Evaluation of a Mycobacterium avium subsp. paratuberculosis leuD mutant as a vaccine candidate against challenge in a caprine model
- PMID: 23408524
- PMCID: PMC3623397
- DOI: 10.1128/CVI.00653-12
Evaluation of a Mycobacterium avium subsp. paratuberculosis leuD mutant as a vaccine candidate against challenge in a caprine model
Abstract
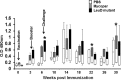
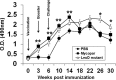
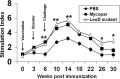
Johne's disease (JD) is prevalent worldwide and has a significant impact on the global agricultural economy. In the present study, we evaluated the protective efficacy of a leuD (Δleud) mutant and gained insight into differential immune responses after challenge with virulent M. avium subsp. paratuberculosis in a caprine colonization model. The immune response and protective efficacy were compared with those of the killed vaccine Mycopar. In vitro stimulation of peripheral blood mononuclear cells with johnin purified protein derivative showed that Mycopar and ΔleuD generated similar levels of gamma interferon (IFN-γ) but significantly higher levels than unvaccinated and challenged phosphate-buffered saline controls. However, only with ΔleuD was the IFN-γ response maintained. Flow cytometric analysis showed that the increase in IFN-γ correlated with proliferation and activation (increased expression of CD25) of CD4, CD8, and γδT cells, but this response was significantly higher in ΔleuD-vaccinated animals at some time points after challenge. Both Mycopar and ΔleuD vaccines upregulated Th1/proinflammatory and Th17 cytokines and downregulated Th2/anti-inflammatory and regulatory cytokines at similar levels at almost all time points. However, significantly higher levels of IFN-γ (at weeks 26 and 30), interleukin-2 (IL-2; week 18), IL-1b (weeks 14 and 22), IL-17 (weeks 18 and 22), and IL-23 (week 18) and a significantly lower level of IL-10 (weeks 14 and 18) and transforming growth factor β (week 18) were detected in the ΔleuD-vaccinated group. Most importantly, ΔleuD elicited an immune response that significantly limited colonization of tissues compared to Mycopar upon challenge with wild-type M. avium subsp. paratuberculosis. In conclusion, the ΔleuD mutant is a promising vaccine candidate for development of a live attenuated vaccine for JD in ruminants.
Figures

References
-
- Sohal JS, Singh SV, Singh PK, Singh AV. 2010. On the evolution of ‘Indian Bison type’ strains of Mycobacterium avium subspecies paratuberculosis. Microbiol. Res. 165: 163–171 - PubMed
-
- Sleeman JM, Manning EJ, Rohm JH, Sims JP, Sanchez S, Gerhold RW, Keel MK. 2009. Johne's disease in a free-ranging white-tailed deer from Virginia and subsequent surveillance for Mycobacterium avium subspecies paratuberculosis. J. Wildl. Dis. 45: 201–206 - PubMed
-
- Clarke CJ. 1997. The pathology and pathogenesis of paratuberculosis in ruminants and other species. J. Comp. Pathol. 116: 217–261 - PubMed
-
- Johnson-Ifearulundu Y, Kaneene JB, Lloyd JW. 1999. Herd-level economic analysis of the impact of paratuberculosis on dairy herds. J. Am. Vet. Med. Assoc. 214: 822–825 - PubMed
-
- Kirkwood CD, Wagner J, Boniface K, Vaughan J, Michalski WP, Catto-Smith AG, Cameron DJ, Bishop RF. 2009. Mycobacterium avium subspecies paratuberculosis in children with early-onset Crohn's disease. Inflamm. Bowel Dis. 15: 1643–1655 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

