Nuclear localization of dengue virus nonstructural protein 5 does not strictly correlate with efficient viral RNA replication and inhibition of type I interferon signaling
- PMID: 23408610
- PMCID: PMC3624364
- DOI: 10.1128/JVI.03083-12
Nuclear localization of dengue virus nonstructural protein 5 does not strictly correlate with efficient viral RNA replication and inhibition of type I interferon signaling
Abstract
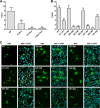
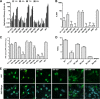
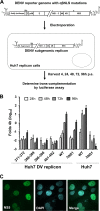
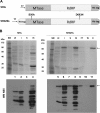
Dengue virus (DENV) is an important human pathogen, especially in the tropical and subtropical parts of the world, causing considerable morbidity and mortality. DENV replication occurs in the cytoplasm; however, a high proportion of nonstructural protein 5 (NS5), containing methyltransferase (MTase) and RNA-dependent RNA polymerase (RdRp) activities, accumulates in the nuclei of infected cells. The present study investigates the impact of nuclear localization of NS5 on its known functions, including viral RNA replication and subversion of the type I interferon response. By using a mutation analysis approach, we identified the most critical residues within the αβ nuclear localization signal (αβNLS), which are essential for the nuclear accumulation of this protein. Although we observed an overall correlation between reduced nuclear accumulation of NS5 and impaired RNA replication, we identified one mutant with drastically reduced amounts of nuclear NS5 and virtually unaffected RNA replication, arguing that nuclear localization of NS5 does not correlate strictly with DENV replication, at least in cell culture. Because NS5 plays an important role in blocking interferon signaling via STAT-2 (signal transducer and activator of transcription 2) degradation, the abilities of the NLS mutants to block this pathway were investigated. All mutants were able to degrade STAT-2, with accordingly similar type I interferon resistance phenotypes. Since the NLS is contained within the RdRp domain, the MTase and RdRp activities of the mutants were determined by using recombinant full-length NS5. We found that the C-terminal region of the αβNLS is a critical functional element of the RdRp domain required for polymerase activity. These results indicate that efficient DENV RNA replication requires only minimal, if any, nuclear NS5, and they identify the αβNLS as a structural element required for proper RdRp activity.
Figures




References
-
- Kyle JL, Harris E. 2008. Global spread and persistence of dengue. Annu. Rev. Microbiol. 62:71–92 - PubMed
-
- Halstead SB.2007. Dengue. Lancet 370:1644–1652 - PubMed
-
- Guy B, Almond JW. 2008. Towards a dengue vaccine: progress to date and remaining challenges. Comp. Immunol. Microbiol. Infect. Dis. 31:239–252 - PubMed
-
- Guy B, Guirakhoo F, Barban V, Higgs S, Monath TP, Lang J. 2010. Preclinical and clinical development of YFV 17D-based chimeric vaccines against dengue, West Nile and Japanese encephalitis viruses. Vaccine 28:632–649 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

