Ganoderma lucidum ethanol extract inhibits the inflammatory response by suppressing the NF-κB and toll-like receptor pathways in lipopolysaccharide-stimulated BV2 microglial cells
- PMID: 23408713
- PMCID: PMC3570243
- DOI: 10.3892/etm.2013.895
Ganoderma lucidum ethanol extract inhibits the inflammatory response by suppressing the NF-κB and toll-like receptor pathways in lipopolysaccharide-stimulated BV2 microglial cells
Abstract
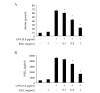
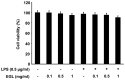
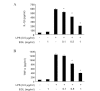
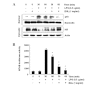
Ganoderma lucidum is a traditional Oriental medicine that has been widely used as a tonic to promote longevity and health in Korea and other Asian countries. Although a great deal of work has been carried out on the therapeutic potential of this mushroom, the pharmacological mechanisms of its anti-inflammatory actions remain unclear. In this study, we evaluated the inhibitory effects of G. lucidum ethanol extract (EGL) on the production of inflammatory mediators and cytokines in lipopolysaccharide (LPS)-stimulated murine BV2 microglia. We also investigated the effects of EGL on the LPS-induced activation of nuclear factor kappaB (NF-κB) and upregulation of toll-like receptor 4 (TLR4) and myeloid differentiation factor 88 (MyD88). Elevated levels of nitric oxide (NO), prostaglandin E(2) (PGE(2)) and pro-inflammatory cytokine production were detected in BV2 microglia following LPS stimulation. We identifed that EGL significantly inhibits the excessive production of NO, PGE(2) and pro-inflammatory cytokines, including interleukin (IL)-1β and tumor necrosis factor-α in a concentration-dependent manner without causing cytotoxicity. In addition, EGL suppressed NF-κB translocation and transcriptional activity by blocking IκB degradation and inhibiting TLR4 and MyD88 expression in LPS-stimulated BV2 cells. Our results indicate that the inhibitory effects of EGL on LPS-stimulated inflammatory responses in BV2 microglia are associated with the suppression of the NF-κB and TLR signaling pathways. Therefore, EGL may be useful in the treatment of neurodegenerative diseases by inhibiting inflammatory mediator responses in activated microglia.
Keywords: BV2 microglia; Ganoderma lucidum; inflammation; nuclear factor κB; toll-like receptor.
Figures



References
-
- Mahajna J, Dotan N, Zaidman BZ, Petrova RD, Wasser SP. Pharmacological values of medicinal mushrooms for prostate cancer therapy: the case of Ganoderma lucidum. Nutr Cancer. 2009;61:16–26. - PubMed
-
- Boh B, Berovic M, Zhang J, Zhi-Bin L. Ganoderma lucidum and its pharmaceutically active compounds. Biotechnol Annu Rev. 2007;13:265–301. - PubMed
-
- Paterson RR. Ganoderma - a therapeutic fungal biofactory. Phytochemistry. 2006;67:1985–2001. - PubMed
-
- Yuen JW, Gohel MD. Anticancer effects of Ganoderma lucidum: a review of scientific evidence. Nutr Cancer. 2005;53:11–17. - PubMed
-
- Chien CM, Cheng JL, Chang WT, Tien MH, Tsao CM, Chang YH, Chang HY, Hsieh JF, Wong CH, Chen ST. Polysaccharides of Ganoderma lucidum alter cell immunophenotypic expression and enhance CD56+ NK-cell cytotoxicity in cord blood. Bioorg Med Chem. 2004;12:5603–5609. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
