Development of an immunoaffinity method for purification of streptokinase
- PMID: 23408770
- PMCID: PMC3558212
Development of an immunoaffinity method for purification of streptokinase
Abstract
Background: Streptokinase is a potent activator of plasminogen to plasmin, the enzyme that can solubilize the fibrin network in blood clots. Streptokinase is currently used in clinical medicine as a thrombolytic agent. It is naturally secreted by β-hemolytic streptococci.
Methods: To reach an efficient method of purification, an immunoaffinity chromatography method was developed that could purify the streptokinase in a single step with high yield. At the first stage, a CNBr-Activated sepharose 4B-Lysine column was made to purify the human blood plasminogen. The purified plasminogen was utilized to construct a column that could purify the streptokinase. The rabbit was immunized with the purified streptokinase and the anti-streptokinase (IgG) purified on another streptokinase substituted sepharose-4B column. The immunoaffinity column was developed by coupling the purified anti-Streptokinase (IgG) to sepharose 6MB-Protein A. The Escherichia coli (E.coli) BL21 (DE3) pLysS strain was transformed by the recombinant construct (cloned streptokinase gene in pGEX-4T-2 vector) and gene expression was induced by IPTG. The expressed protein was purified by immunoaffinity chromatography in a single step.
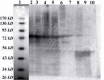
Results: The immunoaffinity column could purify the recombinant fusion GST-SK to homogeneity. The purity of streptokinase was confirmed by SDS-PAGE as a single band of about 71 kD and its biological activity determined in a specific streptokinase assay. The yield of the purification was about 94%.
Conclusion: This method of streptokinase purification is superior to the previous conventional methods.
Keywords: Chromatography; Purification; Streptokinase; Thrombolytic agent.
Figures




References
-
- Banerjee A, Chistic Y, Banerjee UC. Streptokinase, a clinically useful thrombolytic agent. Biotechnol Adv. 2004;22(4):287–307. - PubMed
-
- Castellino FJ. A unique enzyme-protein substrate modifier reaction: plasmin/streptokinase interaction. Trends Biochem Sci. 1979;4(1):1–5.
-
- Rodriguez P, Fuentes P, Barro M, Alvarez JG, Muñoz E, Collen D, et al. Structural domains of streptokinase involved in the interaction with plasminogen. Eur J Biochem. 1995;229(1):83–90. - PubMed
-
- Jackson KW, Tang J. Complete amino acid sequence of streptokinase and its homology with serine proteases. Biochemistry. 1982;21(26):6620–6625. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
