Rv1894c is a novel hypoxia-induced nitronate monooxygenase required for Mycobacterium tuberculosis virulence
- PMID: 23408846
- PMCID: PMC3627198
- DOI: 10.1093/infdis/jit049
Rv1894c is a novel hypoxia-induced nitronate monooxygenase required for Mycobacterium tuberculosis virulence
Abstract
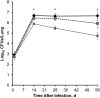
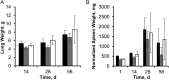
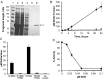
Tuberculosis is difficult to cure, requiring a minimum of 6 months of treatment with multiple antibiotics. Small numbers of organisms are able to tolerate the antibiotics and persist in the lungs of infected humans, but they still require some metabolic activity to survive. We studied the role of the hypoxia-induced Rv1894c gene in Mycobacterium tuberculosis virulence in guinea pigs, which develop hypoxic, necrotic granulomas histologically resembling those in humans and found this gene to be necessary for full bacillary growth and survival. We characterized the function of the encoded enzyme as a nitronate monooxygenase, which is needed to prevent the buildup of toxic products during hypoxic metabolism and is negatively regulated by the transcriptional repressor KstR. Future studies will focus on developing small-molecule inhibitors that target Rv1894c and its homologs, with the goal of killing persistent bacteria, thereby shortening the time needed to treat tuberculosis.
Figures



Similar articles
-
The stringent response is required for full virulence of Mycobacterium tuberculosis in guinea pigs.J Infect Dis. 2010 Nov 1;202(9):1397-404. doi: 10.1086/656524. J Infect Dis. 2010. PMID: 20863231 Free PMC article.
-
Co-expression of DevR and DevR(N)-Aph proteins is associated with hypoxic adaptation defect and virulence attenuation of Mycobacterium tuberculosis.PLoS One. 2010 Feb 26;5(2):e9448. doi: 10.1371/journal.pone.0009448. PLoS One. 2010. PMID: 20195478 Free PMC article.
-
Examination of Mycobacterium tuberculosis sigma factor mutants using low-dose aerosol infection of guinea pigs suggests a role for SigC in pathogenesis.Microbiology (Reading). 2006 Jun;152(Pt 6):1591-1600. doi: 10.1099/mic.0.28591-0. Microbiology (Reading). 2006. PMID: 16735723
-
Hypoxia: a window into Mycobacterium tuberculosis latency.Cell Microbiol. 2009 Aug;11(8):1151-9. doi: 10.1111/j.1462-5822.2009.01325.x. Epub 2009 Apr 15. Cell Microbiol. 2009. PMID: 19388905 Review.
-
Lipid hydrolizing enzymes in virulence: Mycobacterium tuberculosis as a model system.Crit Rev Microbiol. 2010 Aug;36(3):259-69. doi: 10.3109/1040841X.2010.482923. Crit Rev Microbiol. 2010. PMID: 20500016 Review.
Cited by
-
Antibiotic Treatment Shapes the Antigenic Environment During Chronic TB Infection, Offering Novel Targets for Therapeutic Vaccination.Front Immunol. 2020 Apr 28;11:680. doi: 10.3389/fimmu.2020.00680. eCollection 2020. Front Immunol. 2020. PMID: 32411131 Free PMC article.
-
Pseudomonas aeruginosa LysR PA4203 regulator NmoR acts as a repressor of the PA4202 nmoA gene, encoding a nitronate monooxygenase.J Bacteriol. 2015 Mar;197(6):1026-39. doi: 10.1128/JB.01991-14. Epub 2014 Nov 10. J Bacteriol. 2015. PMID: 25384477 Free PMC article.
-
Impact of Hypoxia on Drug Resistance and Growth Characteristics of Mycobacterium tuberculosis Clinical Isolates.PLoS One. 2016 Nov 11;11(11):e0166052. doi: 10.1371/journal.pone.0166052. eCollection 2016. PLoS One. 2016. PMID: 27835653 Free PMC article.
-
Understanding the Role of Nitronate Monooxygenases in Virulence of the Human Fungal Pathogen Aspergillus fumigatus.J Fungi (Basel). 2022 Jul 16;8(7):736. doi: 10.3390/jof8070736. J Fungi (Basel). 2022. PMID: 35887491 Free PMC article.
-
Identification of a Transcription Factor That Regulates Host Cell Exit and Virulence of Mycobacterium tuberculosis.PLoS Pathog. 2016 May 18;12(5):e1005652. doi: 10.1371/journal.ppat.1005652. eCollection 2016 May. PLoS Pathog. 2016. PMID: 27191591 Free PMC article.
References
-
- Haapanen JH, Kass I, Gensini G, Middlebrook G. Studies on the gaseous content of tuberculous cavities. Am Rev Respir Dis. 1959;80:1–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

