CDX2 regulation by the RNA-binding protein MEX3A: impact on intestinal differentiation and stemness
- PMID: 23408853
- PMCID: PMC3627580
- DOI: 10.1093/nar/gkt087
CDX2 regulation by the RNA-binding protein MEX3A: impact on intestinal differentiation and stemness
Abstract
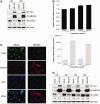
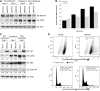
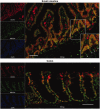
The homeobox transcription factor CDX2 plays a crucial role in intestinal cell fate specification, both during normal development and in tumorigenic processes involving intestinal reprogramming. The CDX2 regulatory network is intricate, but it has not yet been fully uncovered. Through genome-wide screening of a 3D culture system, the RNA-binding protein MEX3A was identified as putatively involved in CDX2 regulation; therefore, its biological relevance was addressed by setting up cell-based assays together with expression studies in murine intestine. We demonstrate here that MEX3A has a repressive function by controlling CDX2 levels in gastric and colorectal cellular models. This is dependent on the interaction with a specific binding determinant present in CDX2 mRNA 3'untranslated region. We have further determined that MEX3A impairs intestinal differentiation and cellular polarization, affects cell cycle progression and promotes increased expression of intestinal stem cell markers, namely LGR5, BMI1 and MSI1. Finally, we show that MEX3A is expressed in mouse intestine, supporting an in vivo context for interaction with CDX2 and modulation of stem cell properties. Therefore, we describe a novel CDX2 post-transcriptional regulatory mechanism, through the RNA-binding protein MEX3A, with a major impact in intestinal differentiation, polarity and stemness, likely contributing to intestinal homeostasis and carcinogenesis.
Figures





Similar articles
-
Cooperation between HNF-1alpha, Cdx2, and GATA-4 in initiating an enterocytic differentiation program in a normal human intestinal epithelial progenitor cell line.Am J Physiol Gastrointest Liver Physiol. 2010 Apr;298(4):G504-17. doi: 10.1152/ajpgi.00265.2009. Epub 2010 Feb 4. Am J Physiol Gastrointest Liver Physiol. 2010. PMID: 20133952 Free PMC article.
-
SMYD2 epigenetically activates MEX3A and suppresses CDX2 in colorectal cancer cells to augment cancer growth.Clin Exp Pharmacol Physiol. 2022 Sep;49(9):959-969. doi: 10.1111/1440-1681.13679. Epub 2022 Jun 18. Clin Exp Pharmacol Physiol. 2022. PMID: 35637161
-
Expression and localisation of insulin receptor substrate 2 in normal intestine and colorectal tumours. Regulation by intestine-specific transcription factor CDX2.Gut. 2009 Sep;58(9):1250-9. doi: 10.1136/gut.2008.158386. Epub 2009 Feb 15. Gut. 2009. PMID: 19221108
-
The role of CDX2 in inflammatory bowel disease.Dan Med J. 2014 Mar;61(3):B4820. Dan Med J. 2014. PMID: 24814920 Review.
-
Extending the functions of the homeotic transcription factor Cdx2 in the digestive system through nontranscriptional activities.World J Gastroenterol. 2015 Feb 7;21(5):1436-43. doi: 10.3748/wjg.v21.i5.1436. World J Gastroenterol. 2015. PMID: 25663763 Free PMC article. Review.
Cited by
-
The third dimension: new developments in cell culture models for colorectal research.Cell Mol Life Sci. 2016 Nov;73(21):3971-89. doi: 10.1007/s00018-016-2258-2. Epub 2016 May 4. Cell Mol Life Sci. 2016. PMID: 27147463 Free PMC article. Review.
-
The Stemness Gene Mex3A Is a Key Regulator of Neuroblast Proliferation During Neurogenesis.Front Cell Dev Biol. 2020 Sep 22;8:549533. doi: 10.3389/fcell.2020.549533. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33072742 Free PMC article.
-
Mex-3B induces apoptosis by inhibiting miR-92a access to the Bim-3'UTR.Oncogene. 2018 Sep;37(38):5233-5247. doi: 10.1038/s41388-018-0336-7. Epub 2018 May 30. Oncogene. 2018. PMID: 29849121
-
RNA Binding Proteins in Intestinal Epithelial Biology and Colorectal Cancer.Trends Mol Med. 2018 May;24(5):490-506. doi: 10.1016/j.molmed.2018.03.008. Epub 2018 Apr 5. Trends Mol Med. 2018. PMID: 29627433 Free PMC article. Review.
-
Comprehensive Analysis of Prognostic Value of MEX3A and Its Relationship with Immune Infiltrates in Ovarian Cancer.J Immunol Res. 2021 Jun 3;2021:5574176. doi: 10.1155/2021/5574176. eCollection 2021. J Immunol Res. 2021. PMID: 34189143 Free PMC article.
References
-
- Silberg DG, Swain GP, Suh ER, Traber PG. Cdx1 and cdx2 expression during intestinal development. Gastroenterology. 2000;119:961–971. - PubMed
-
- Gross I, Duluc I, Benameur T, Calon A, Martin E, Brabletz T, Kedinger M, Domon-Dell C, Freund JN. The intestine-specific homeobox gene Cdx2 decreases mobility and antagonizes dissemination of colon cancer cells. Oncogene. 2008;27:107–115. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

