P53-mediated rapid induction of apoptosis conveys resistance to viral infection in Drosophila melanogaster
- PMID: 23408884
- PMCID: PMC3567152
- DOI: 10.1371/journal.ppat.1003137
P53-mediated rapid induction of apoptosis conveys resistance to viral infection in Drosophila melanogaster
Abstract
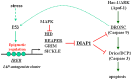
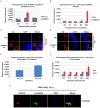
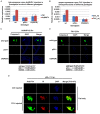
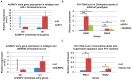
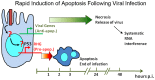
Arthropod-borne pathogens account for millions of deaths each year. Understanding the genetic mechanisms controlling vector susceptibility to pathogens has profound implications for developing novel strategies for controlling insect-transmitted infectious diseases. The fact that many viruses carry genes that have anti-apoptotic activity has long led to the hypothesis that induction of apoptosis could be a fundamental innate immune response. However, the cellular mechanisms mediating the induction of apoptosis following viral infection remained enigmatic, which has prevented experimental verification of the functional significance of apoptosis in limiting viral infection in insects. In addition, studies with cultured insect cells have shown that there is sometimes a lack of apoptosis, or the pro-apoptotic response happens relatively late, thus casting doubt on the functional significance of apoptosis as an innate immunity. Using in vivo mosquito models and the native route of infection, we found that there is a rapid induction of reaper-like pro-apoptotic genes within a few hours following exposure to DNA or RNA viruses. Recapitulating a similar response in Drosophila, we found that this rapid induction of apoptosis requires the function of P53 and is mediated by a stress-responsive regulatory region upstream of reaper. More importantly, we showed that the rapid induction of apoptosis is responsible for preventing the expression of viral genes and blocking the infection. Genetic changes influencing this rapid induction of reaper-like pro-apoptotic genes led to significant differences in susceptibility to viral infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Clouston WM, Kerr JF (1985) Apoptosis, lymphocytotoxicity and the containment of viral infections. Med Hypotheses 18: 399–404. - PubMed
-
- Hardwick JM (1998) Viral interference with apoptosis. Semin Cell Dev Biol 9: 339–349. - PubMed
-
- Everett H, McFadden G (1999) Apoptosis: an innate immune response to virus infection. Trends Microbiol 7: 160–165. - PubMed
-
- Benedict CA, Norris PS, Ware CF (2002) To kill or be killed: viral evasion of apoptosis. Nat Immunol 3: 1013–1018. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

