Human monoclonal antibodies broadly neutralizing against influenza B virus
- PMID: 23408886
- PMCID: PMC3567173
- DOI: 10.1371/journal.ppat.1003150
Human monoclonal antibodies broadly neutralizing against influenza B virus
Abstract
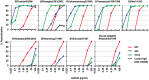
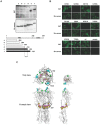
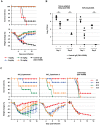
Influenza virus has the ability to evade host immune surveillance through rapid viral genetic drift and reassortment; therefore, it remains a continuous public health threat. The development of vaccines producing broadly reactive antibodies, as well as therapeutic strategies using human neutralizing monoclonal antibodies (HuMAbs) with global reactivity, has been gathering great interest recently. Here, three hybridoma clones producing HuMAbs against influenza B virus, designated 5A7, 3A2 and 10C4, were prepared using peripheral lymphocytes from vaccinated volunteers, and were investigated for broad cross-reactive neutralizing activity. Of these HuMAbs, 3A2 and 10C4, which recognize the readily mutable 190-helix region near the receptor binding site in the hemagglutinin (HA) protein, react only with the Yamagata lineage of influenza B virus. By contrast, HuMAb 5A7 broadly neutralizes influenza B strains that were isolated from 1985 to 2006, belonging to both Yamagata and Victoria lineages. Epitope mapping revealed that 5A7 recognizes 316G, 318C and 321W near the C terminal of HA1, a highly conserved region in influenza B virus. Indeed, no mutations in the amino acid residues of the epitope region were induced, even after the virus was passaged ten times in the presence of HuMAb 5A7. Moreover, 5A7 showed significant therapeutic efficacy in mice, even when it was administered 72 hours post-infection. These results indicate that 5A7 is a promising candidate for developing therapeutics, and provide insight for the development of a universal vaccine against influenza B virus.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Conserved neutralizing epitope at globular head of hemagglutinin in H3N2 influenza viruses.J Virol. 2014 Jul;88(13):7130-44. doi: 10.1128/JVI.00420-14. Epub 2014 Apr 9. J Virol. 2014. PMID: 24719430 Free PMC article.
-
A human antibody recognizing a conserved epitope of H5 hemagglutinin broadly neutralizes highly pathogenic avian influenza H5N1 viruses.J Virol. 2012 Mar;86(6):2978-89. doi: 10.1128/JVI.06665-11. Epub 2012 Jan 11. J Virol. 2012. PMID: 22238297 Free PMC article.
-
Mapping of a Novel H3-Specific Broadly Neutralizing Monoclonal Antibody Targeting the Hemagglutinin Globular Head Isolated from an Elite Influenza Virus-Immunized Donor Exhibiting Serological Breadth.J Virol. 2020 Feb 28;94(6):e01035-19. doi: 10.1128/JVI.01035-19. Print 2020 Feb 28. J Virol. 2020. PMID: 31826999 Free PMC article.
-
Broad-range neutralizing anti-influenza A human monoclonal antibodies: new perspectives in therapy and prophylaxis.New Microbiol. 2012 Oct;35(4):399-406. Epub 2012 Oct 1. New Microbiol. 2012. PMID: 23109007 Review.
-
Recent advances in "universal" influenza virus antibodies: the rise of a hidden trimeric interface in hemagglutinin globular head.Front Med. 2020 Apr;14(2):149-159. doi: 10.1007/s11684-020-0764-y. Epub 2020 Apr 1. Front Med. 2020. PMID: 32239416 Free PMC article. Review.
Cited by
-
A small-molecule fusion inhibitor of influenza virus is orally active in mice.Science. 2019 Mar 8;363(6431):eaar6221. doi: 10.1126/science.aar6221. Science. 2019. PMID: 30846569 Free PMC article.
-
A broadly protective therapeutic antibody against influenza B virus with two mechanisms of action.Nat Commun. 2017 Jan 19;8:14234. doi: 10.1038/ncomms14234. Nat Commun. 2017. PMID: 28102191 Free PMC article.
-
Human Monoclonal Antibody Derived from Transchromosomic Cattle Neutralizes Multiple H1 Clades of Influenza A Virus by Recognizing a Novel Conformational Epitope in the Hemagglutinin Head Domain.J Virol. 2020 Oct 27;94(22):e00945-20. doi: 10.1128/JVI.00945-20. Print 2020 Oct 27. J Virol. 2020. PMID: 32847862 Free PMC article.
-
Influenza: A broadly protective antibody.Nature. 2017 Nov 15;551(7680):310-311. doi: 10.1038/551310a. Nature. 2017. PMID: 29144466 No abstract available.
-
Insights from the comparison of genomic variants from two influenza B viruses grown in the presence of human antibodies in cell culture.PLoS One. 2020 Sep 14;15(9):e0239015. doi: 10.1371/journal.pone.0239015. eCollection 2020. PLoS One. 2020. PMID: 32925936 Free PMC article.
References
-
- Lambert LC, Fauci AS (2010) Influenza vaccines for the future. N Engl J Med 363: 2036–2044. - PubMed
-
- WHO (2009) Influenza (Seasonal). Available: http://www.who.int/mediacentre/factsheets/fs211/en/.
-
- Carrat F, Flahault A (2007) Influenza vaccine: the challenge of antigenic drift. Vaccine 25: 6852–6862. - PubMed
-
- Webster RG, Berton MT (1981) Analysis of antigenic drift in the haemagglutinin molecule of influenza B virus with monoclonal antibodies. J Gen Virol 54: 243–251. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

