Regulation of Neurod1 contributes to the lineage potential of Neurogenin3+ endocrine precursor cells in the pancreas
- PMID: 23408910
- PMCID: PMC3567185
- DOI: 10.1371/journal.pgen.1003278
Regulation of Neurod1 contributes to the lineage potential of Neurogenin3+ endocrine precursor cells in the pancreas
Abstract
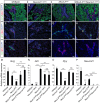
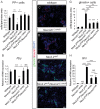
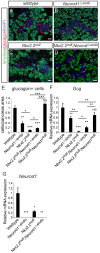
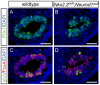
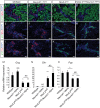
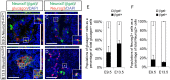
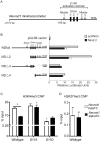
During pancreatic development, transcription factor cascades gradually commit precursor populations to the different endocrine cell fate pathways. Although mutational analyses have defined the functions of many individual pancreatic transcription factors, the integrative transcription factor networks required to regulate lineage specification, as well as their sites of action, are poorly understood. In this study, we investigated where and how the transcription factors Nkx2.2 and Neurod1 genetically interact to differentially regulate endocrine cell specification. In an Nkx2.2 null background, we conditionally deleted Neurod1 in the Pdx1+ pancreatic progenitor cells, the Neurog3+ endocrine progenitor cells, or the glucagon+ alpha cells. These studies determined that, in the absence of Nkx2.2 activity, removal of Neurod1 from the Pdx1+ or Neurog3+ progenitor populations is sufficient to reestablish the specification of the PP and epsilon cell lineages. Alternatively, in the absence of Nkx2.2, removal of Neurod1 from the Pdx1+ pancreatic progenitor population, but not the Neurog3+ endocrine progenitor cells, restores alpha cell specification. Subsequent in vitro reporter assays demonstrated that Nkx2.2 represses Neurod1 in alpha cells. Based on these findings, we conclude that, although Nkx2.2 and Neurod1 are both necessary to promote beta cell differentiation, Nkx2.2 must repress Neurod1 in a Pdx1+ pancreatic progenitor population to appropriately commit a subset of Neurog3+ endocrine progenitor cells to the alpha cell lineage. These results are consistent with the proposed idea that Neurog3+ endocrine progenitor cells represent a heterogeneous population of unipotent cells, each restricted to a particular endocrine lineage.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Cooperative transcriptional regulation of the essential pancreatic islet gene NeuroD1 (beta2) by Nkx2.2 and neurogenin 3.J Biol Chem. 2009 Nov 6;284(45):31236-48. doi: 10.1074/jbc.M109.048694. Epub 2009 Sep 15. J Biol Chem. 2009. PMID: 19759004 Free PMC article.
-
Genetic identification of a novel NeuroD1 function in the early differentiation of islet alpha, PP and epsilon cells.Dev Biol. 2007 Dec 15;312(2):523-32. doi: 10.1016/j.ydbio.2007.09.057. Epub 2007 Oct 5. Dev Biol. 2007. PMID: 17988662 Free PMC article.
-
NEUROD1 Is Required for the Early α and β Endocrine Differentiation in the Pancreas.Int J Mol Sci. 2021 Jun 23;22(13):6713. doi: 10.3390/ijms22136713. Int J Mol Sci. 2021. PMID: 34201511 Free PMC article.
-
PDX1, Neurogenin-3, and MAFA: critical transcription regulators for beta cell development and regeneration.Stem Cell Res Ther. 2017 Nov 2;8(1):240. doi: 10.1186/s13287-017-0694-z. Stem Cell Res Ther. 2017. PMID: 29096722 Free PMC article. Review.
-
Revisiting the immunocytochemical detection of Neurogenin 3 expression in mouse and man.Diabetes Obes Metab. 2016 Sep;18 Suppl 1:10-22. doi: 10.1111/dom.12718. Diabetes Obes Metab. 2016. PMID: 27615127 Review.
Cited by
-
Transcription factor regulation of pancreatic organogenesis, differentiation and maturation.Islets. 2016;8(1):13-34. doi: 10.1080/19382014.2015.1075687. Epub 2015 Sep 24. Islets. 2016. PMID: 26404721 Free PMC article. Review.
-
Protocol for in vitro modeling of specification and morphogenesis of early pancreas development using human pluripotent stem cell-based organoid differentiation.STAR Protoc. 2025 Jun 20;6(2):103834. doi: 10.1016/j.xpro.2025.103834. Epub 2025 May 21. STAR Protoc. 2025. PMID: 40402748 Free PMC article.
-
Deoxyhypusine synthase, an essential enzyme for hypusine biosynthesis, is required for proper exocrine pancreas development.FASEB J. 2021 May;35(5):e21473. doi: 10.1096/fj.201903177R. FASEB J. 2021. PMID: 33811703 Free PMC article.
-
NEUROD1: transcriptional and epigenetic regulator of human and mouse neuronal and endocrine cell lineage programs.Front Cell Dev Biol. 2024 Jul 22;12:1435546. doi: 10.3389/fcell.2024.1435546. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39105169 Free PMC article. Review.
-
A Specialized Niche in the Pancreatic Microenvironment Promotes Endocrine Differentiation.Dev Cell. 2020 Oct 26;55(2):150-162.e6. doi: 10.1016/j.devcel.2020.08.003. Epub 2020 Aug 27. Dev Cell. 2020. PMID: 32857951 Free PMC article.
References
-
- D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, et al. (2006) Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 24: 1392–1401. - PubMed
-
- Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, et al. (2008) Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol 26: 443–452. - PubMed
-
- Gu G, Dubauskaite J, Melton DA (2002) Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 129: 2447–2457. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

