Mitotic evolution of Plasmodium falciparum shows a stable core genome but recombination in antigen families
- PMID: 23408914
- PMCID: PMC3567157
- DOI: 10.1371/journal.pgen.1003293
Mitotic evolution of Plasmodium falciparum shows a stable core genome but recombination in antigen families
Abstract
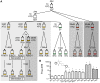
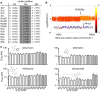
Malaria parasites elude eradication attempts both within the human host and across nations. At the individual level, parasites evade the host immune responses through antigenic variation. At the global level, parasites escape drug pressure through single nucleotide variants and gene copy amplification events conferring drug resistance. Despite their importance to global health, the rates at which these genomic alterations emerge have not been determined. We studied the complete genomes of different Plasmodium falciparum clones that had been propagated asexually over one year in the presence and absence of drug pressure. A combination of whole-genome microarray analysis and next-generation deep resequencing (totaling 14 terabases) revealed a stable core genome with only 38 novel single nucleotide variants appearing in seventeen evolved clones (avg. 5.4 per clone). In clones exposed to atovaquone, we found cytochrome b mutations as well as an amplification event encompassing the P. falciparum multidrug resistance associated protein (mrp1) on chromosome 1. We observed 18 large-scale (>1 kb on average) deletions of telomere-proximal regions encoding multigene families, involved in immune evasion (9.5×10(-6) structural variants per base pair per generation). Six of these deletions were associated with chromosomal crossovers generated during mitosis. We found only minor differences in rates between genetically distinct strains and between parasites cultured in the presence or absence of drug. Using these derived mutation rates for P. falciparum (1.0-9.7×10(-9) mutations per base pair per generation), we can now model the frequency at which drug or immune resistance alleles will emerge under a well-defined set of assumptions. Further, the detection of mitotic recombination events in var gene families illustrates how multigene families can arise and change over time in P. falciparum. These results will help improve our understanding of how P. falciparum evolves to evade control efforts within both the individual hosts and large populations.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- WHO (2011) World Malaria Report 2011. http://www.who.int/malaria/world_malaria_report_2010/en/index.html.
-
- Genton B, Betuela I, Felger I, Al-Yaman F, Anders RF, et al. (2002) A recombinant blood-stage malaria vaccine reduces Plasmodium falciparum density and exerts selective pressure on parasite populations in a phase 1–2b trial in Papua New Guinea. The Journal of Infectious Diseases 185: 820–827. - PubMed
-
- Freitas-Junior LH, Bottius E, Pirrit LA, Deitsch KW, Scheidig C, et al. (2000) Frequent ectopic recombination of virulence factor genes in telomeric chromosome clusters of P. falciparum. Nature 407: 1018–1022. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

