Glutamate receptor subunit and calmodulin kinase II expression, with and without T maze training, in the rat hippocampus following bilateral vestibular deafferentation
- PMID: 23408944
- PMCID: PMC3567128
- DOI: 10.1371/journal.pone.0054527
Glutamate receptor subunit and calmodulin kinase II expression, with and without T maze training, in the rat hippocampus following bilateral vestibular deafferentation
Abstract
Many previous studies have shown that lesions of the peripheral vestibular system result in spatial memory deficits and electrophysiological dysfunction in the hippocampus. Given the importance of glutamate as a neurotransmitter in the hippocampus, it was predicted that bilateral vestibular deafferentation (BVD) would alter the expression of NMDA and AMPA receptors in this area of the brain.
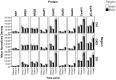
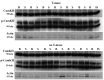
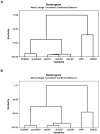
Methods: The expression of the NR1, NR2A, NR2B, GluR1, GluR2, GluR3 and GluR 4 glutamate receptor subunits, as well as calmodulin kinase IIα (CaMKIIα) and phosphorylated CaMKIIα (pCaMKIIα), was measured in the rat CA1, CA2/3 and dentate gyrus (DG) subregions of the hippocampus, at 24 h, 72 h, 1 week, 1 month and 6 months following BVD, using western blotting. In the 6 month group, half of the animals underwent spatial forced alternating training in a T-maze.
Results and discussion: For the 24 h, 72 h, 1 week and 1 month data, there was no significant effect of surgery for any hippocampal subregion. However, for the 6 month data set, T maze training had a significant effect independently of surgery. The results of these experiments suggest that BVD is not associated with large changes in glutamate receptor subunit or CaMKIIα expression in the rat hippocampus, at least in terms of both the intra-cytoplasmic and membrane receptor subunits together, that western blotting can measure. However, spatial training-associated increases in glutamate receptor and CaMKIIα expression can be induced in BVD rats with impaired spatial performance. Therefore, the neurophysiological changes underlying BVD-induced spatial learning and memory deficits are more likely to be due to up and down regulation or changes in affinity/efficacy of glutamate receptors at the membrane level than changes in subunit transcription and transduction at the intra-cytoplasmic level.
Conflict of interest statement
Figures


Similar articles
-
Principal component analysis suggests subtle changes in glutamate receptor subunit expression in the rat hippocampus following bilateral vestibular deafferentation.Neurosci Lett. 2013 Aug 26;548:265-8. doi: 10.1016/j.neulet.2013.05.036. Epub 2013 May 27. Neurosci Lett. 2013. PMID: 23721785
-
Gestational nicotine exposure regulates expression of AMPA and NMDA receptors and their signaling apparatus in developing and adult rat hippocampus.Neuroscience. 2011 Aug 11;188:168-81. doi: 10.1016/j.neuroscience.2011.04.069. Epub 2011 May 12. Neuroscience. 2011. PMID: 21596105 Free PMC article.
-
Modification of ionotropic glutamate receptor-mediated processes in the rat hippocampus following repeated, brief seizures.Neuroscience. 2009 Mar 3;159(1):358-68. doi: 10.1016/j.neuroscience.2008.12.027. Epub 2008 Dec 27. Neuroscience. 2009. PMID: 19154779
-
Long-term changes in hippocampal n-methyl-D-aspartate receptor subunits following unilateral vestibular damage in rat.Neuroscience. 2003;117(4):965-70. doi: 10.1016/s0306-4522(02)00878-3. Neuroscience. 2003. PMID: 12654348
-
The Effects of Complete Vestibular Deafferentation on Spatial Memory and the Hippocampus in the Rat: The Dunedin Experience.Multisens Res. 2015;28(5-6):461-85. doi: 10.1163/22134808-00002469. Multisens Res. 2015. PMID: 26595952 Review.
Cited by
-
On the Application of Multivariate Statistical and Data Mining Analyses to Data in Neuroscience.J Undergrad Neurosci Educ. 2018 Jun 15;16(2):R20-R32. eCollection 2018 Spring. J Undergrad Neurosci Educ. 2018. PMID: 30057506 Free PMC article. Review.
-
Spatial cognition, body representation and affective processes: the role of vestibular information beyond ocular reflexes and control of posture.Front Integr Neurosci. 2014 May 27;8:44. doi: 10.3389/fnint.2014.00044. eCollection 2014. Front Integr Neurosci. 2014. PMID: 24904327 Free PMC article. Review.
-
From ear to uncertainty: vestibular contributions to cognitive function.Front Integr Neurosci. 2013 Nov 26;7:84. doi: 10.3389/fnint.2013.00084. Front Integr Neurosci. 2013. PMID: 24324413 Free PMC article. Review.
-
Applications of Multivariate Statistical and Data Mining Analyses to the Search for Biomarkers of Sensorineural Hearing Loss, Tinnitus, and Vestibular Dysfunction.Front Neurol. 2021 Mar 3;12:627294. doi: 10.3389/fneur.2021.627294. eCollection 2021. Front Neurol. 2021. PMID: 33746881 Free PMC article.
-
Cognitive Function in Acquired Bilateral Vestibulopathy: A Cross-Sectional Study on Cognition, Hearing, and Vestibular Loss.Front Neurosci. 2019 Apr 24;13:340. doi: 10.3389/fnins.2019.00340. eCollection 2019. Front Neurosci. 2019. PMID: 31105513 Free PMC article.
References
-
- Stackman R, Herbert A (2002) Rats with lesions of the vestibular system require a visual landmark for spatial navigation. Behav Brain Res 128: 27–40. - PubMed
-
- Russell N, Horii A, Smith PF, Darlington CL, Bilkey DK (2003) Bilateral peripheral vestibular lesions produce long-term changes in spatial learning in the rat. J Vest Res 13: 9–16. - PubMed
-
- Zheng Y, Darlington C, Smith PF (2006) Impairment and recovery on a food foraging task following unilateral vestibular deafferentation in rats. Hippocampus 16: 368–378. - PubMed
-
- Zheng Y, Goddard M, Darlington C, Smith PF (2007) Bilateral vestibular deafferentation impairs performance in a spatial forced alternation task in rats. Hippocampus 17: 253–256. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

