Genetic and pharmacological modifications of thrombin formation in apolipoprotein e-deficient mice determine atherosclerosis severity and atherothrombosis onset in a neutrophil-dependent manner
- PMID: 23409043
- PMCID: PMC3567111
- DOI: 10.1371/journal.pone.0055784
Genetic and pharmacological modifications of thrombin formation in apolipoprotein e-deficient mice determine atherosclerosis severity and atherothrombosis onset in a neutrophil-dependent manner
Abstract
Background: Variations in the blood coagulation activity, determined genetically or by medication, may alter atherosclerotic plaque progression, by influencing pleiotropic effects of coagulation proteases. Published experimental studies have yielded contradictory findings on the role of hypercoagulability in atherogenesis. We therefore sought to address this matter by extensively investigating the in vivo significance of genetic alterations and pharmacologic inhibition of thrombin formation for the onset and progression of atherosclerosis, and plaque phenotype determination.
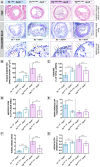
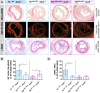
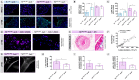
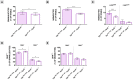
Methodology/principal findings: We generated transgenic atherosclerosis-prone mice with diminished coagulant or hypercoagulable phenotype and employed two distinct models of atherosclerosis. Gene-targeted 50% reduction in prothrombin (FII(-/WT):ApoE(-/-)) was remarkably effective in limiting disease compared to control ApoE(-/-) mice, associated with significant qualitative benefits, including diminished leukocyte infiltration, altered collagen and vascular smooth muscle cell content. Genetically-imposed hypercoagulability in TM(Pro/Pro):ApoE(-/-) mice resulted in severe atherosclerosis, plaque vulnerability and spontaneous atherothrombosis. Hypercoagulability was associated with a pronounced neutrophilia, neutrophil hyper-reactivity, markedly increased oxidative stress, neutrophil intraplaque infiltration and apoptosis. Administration of either the synthetic specific thrombin inhibitor Dabigatran etexilate, or recombinant activated protein C (APC), counteracted the pro-inflammatory and pro-atherogenic phenotype of pro-thrombotic TM(Pro/Pro):ApoE(-/-) mice.
Conclusions/significance: We provide new evidence highlighting the importance of neutrophils in the coagulation-inflammation interplay during atherogenesis. Our findings reveal that thrombin-mediated proteolysis is an unexpectedly powerful determinant of atherosclerosis in multiple distinct settings. These studies suggest that selective anticoagulants employed to prevent thrombotic events may also be remarkably effective in clinically impeding the onset and progression of cardiovascular disease.
Conflict of interest statement
Figures



References
-
- Degen JL, Bugge TH, Goguen JD (2007) Fibrin and fibrinolysis in infection and host defense. Journal of thrombosis and haemostasis : JTH 5 Suppl 124–31. - PubMed
-
- Delvaeye M, Conway EM (2009) Coagulation and innate immune responses: can we view them separately? Blood 114: 2367–2374. - PubMed
-
- Massberg S, Grahl L, von Bruehl ML, Manukyan D, Pfeiler S, et al. (2010) Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nature medicine 16: 887–896. - PubMed
-
- Ross R (1999) Atherosclerosis - an inflammatory disease. N Engl J Med 340: 115–126. - PubMed
-
- Libby P (2002) Inflammation in atherosclerosis. Nature 420: 868–874. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

