Urinary vitamin D binding protein: a potential novel marker of renal interstitial inflammation and fibrosis
- PMID: 23409077
- PMCID: PMC3569442
- DOI: 10.1371/journal.pone.0055887
Urinary vitamin D binding protein: a potential novel marker of renal interstitial inflammation and fibrosis
Abstract
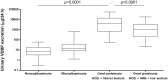
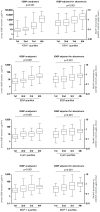
Non-invasive tubulointerstitial damage markers may allow better titration and monitoring of renoprotective therapy. We investigated the value of urinary vitamin D binding protein excretion (uVDBP) as a tubulointerstitial inflammation and fibrosis marker in adriamycin rats, and tested whether uVDBP parallels renal damage and responds to therapy intensification in humans. In adriamycin (ADR) rats, uVDBP was strongly elevated vs controls (CON) already 6 wks after nephrosis induction (ADR: 727±674 [mean±SD] vs CON: 9±12 µg/d, p<0.01), i.e. before onset of pre-fibrotic and inflammatory tubulointerstitial damage, and at all following 6-wk time points until end of follow up at 30 wks (ADR: 1403±1026 vs CON: 206±132 µg/d, p<0.01). In multivariate regression analysis, uVDBP was associated with tubulointerstitial macrophage accumulation (standardized beta = 0.47, p = 0.01) and collagen III expression (standardized beta = 0.44, p = 0.02) independently of albuminuria. In humans, uVDBP was increased in 100 microalbuminuric subjects (44±93 µg/d) and in 47 CKD patients with overt proteinuria (9.2±13.0 mg/d) compared to 100 normoalbuminuric subjects (12±12 µg/d, p<0.001). In CKD patients, uVDBP responded to intensification of renoprotective therapy (ACEi+liberal sodium: 9.2±13.0 mg/d vs dual RAAS blockade+low sodium: 2747±4013, p<0.001), but remained still >100-fold increased during maximal therapy vs normoalbuminurics (p<0.001), consistent with persisting tubulointerstitial damage. UVDBP was associated with tubular and inflammatory damage markers KIM-1 (standardized beta = 0.52, p<0.001), beta-2-microglobuline (st.beta = 0.45, p<0.001), cystatin C (st.beta = 0.40, p<0.001), MCP-1 (st.beta = 0.31, p<0.001) and NGAL (st.beta = 0.20, p = 0.005), independently of albuminuria. UVDBP may be a novel urinary biomarker of tubulointerstitial damage. Prospectively designed studies are required to validate our findings and confirm its relevance in the clinical setting.
Conflict of interest statement
Figures


References
-
- Perico N, Cattaneo D, Remuzzi G (2009) Kidney injury molecule 1: In search of biomarkers of chronic tubulointerstitial damage and disease progression. Am J Kidney Dis 53: 1–4. - PubMed
-
- Verboven C, Rabijns A, De Maeyer M, Van Baelen H, Bouillon R, et al. (2002) A structural basis for the unique binding features of the human vitamin D-binding protein. Nat Struct Biol 9: 131–136. - PubMed
-
- Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, et al. (1999) An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell 96: 507–515. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

