In vivo imaging with fluorescent smart probes to assess treatment strategies for acute pancreatitis
- PMID: 23409095
- PMCID: PMC3569412
- DOI: 10.1371/journal.pone.0055959
In vivo imaging with fluorescent smart probes to assess treatment strategies for acute pancreatitis
Abstract
Background and aims: Endoprotease activation is a key step in acute pancreatitis and early inhibition of these enzymes may protect from organ damage. In vivo models commonly used to evaluate protease inhibitors require animal sacrifice and therefore limit the assessment of dynamic processes. Here, we established a non-invasive fluorescence imaging-based biomarker assay to assess real-time protease inhibition and disease progression in a preclinical model of experimental pancreatitis.
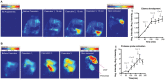
Methods: Edema development and trypsin activation were imaged in a rat caerulein-injection pancreatitis model. A fluorescent "smart" probe, selectively activated by trypsin, was synthesized by labeling with Cy5.5 of a pegylated poly-L-lysine copolymer. Following injection of the probe, trypsin activation was monitored in the presence or absence of inhibitors by in vivo and ex vivo imaging.
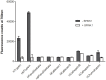
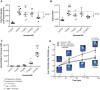
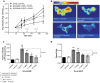
Results: We established the trypsin-selectivity of the fluorescent probe in vitro using a panel of endopeptidases and specific inhibitor. In vivo, the probe accumulated in the liver and a region attributed to the pancreas by necropsy. A dose dependent decrease of total pancreatic fluorescence signal occurred upon administration of known trypsin inhibitors. The fluorescence-based method was a better predictor of trypsin inhibition than pancreatic to body weight ratio.
Conclusions: We established a fluorescence imaging assay to access trypsin inhibition in real-time in vivo. This method is more sensitive and dynamic than classic tissue sample readouts and could be applied to preclinically optimize trypsin inhibitors towards intrapancreatic target inhibition.
Conflict of interest statement
Figures


References
-
- Rickes S, Uhle C (2009) Advances in the diagnosis of acute pancreatitis. Postgrad Med J 85: 208–212. - PubMed
-
- Kylanpaa-Back ML, Kemppainen E, Puolakkainen P (2002) Trypsin-based laboratory methods and carboxypeptidase activation peptide in acute pancreatitis. JOP 3: 34–48. - PubMed
-
- Spanier BW, Dijkgraaf MG, Bruno MJ (2008) Epidemiology, aetiology and outcome of acute and chronic pancreatitis: An update. Best Pract Res Clin Gastroenterol 22: 45–63. - PubMed
-
- Carroll JK, Herrick B, Gipson T, Lee SP (2007) Acute pancreatitis: diagnosis, prognosis, and treatment. Am Fam Physician 75: 1513–1520. - PubMed
-
- Le Marechal C, Masson E, Chen JM, Morel F, Ruszniewski P, et al. (2006) Hereditary pancreatitis caused by triplication of the trypsinogen locus. Nat Genet 38: 1372–1374. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

