Deletion of fibroblast growth factor receptor 2 from the peri-wolffian duct stroma leads to ureteric induction abnormalities and vesicoureteral reflux
- PMID: 23409123
- PMCID: PMC3567073
- DOI: 10.1371/journal.pone.0056062
Deletion of fibroblast growth factor receptor 2 from the peri-wolffian duct stroma leads to ureteric induction abnormalities and vesicoureteral reflux
Erratum in
-
Correction: Deletion of Fibroblast Growth Factor Receptor 2 from the Peri-Wolffian Duct Stroma Leads to Ureteric Induction Abnormalities and Vesicoureteral Reflux.PLoS One. 2016 Nov 18;11(11):e0167191. doi: 10.1371/journal.pone.0167191. eCollection 2016. PLoS One. 2016. PMID: 27861580 Free PMC article.
Abstract
Purpose: Pax3cre-mediated deletion of fibroblast growth factor receptor 2 (Fgfr2) broadly in renal and urinary tract mesenchyme led to ureteric bud (UB) induction defects and vesicoureteral reflux (VUR), although the mechanisms were unclear. Here, we investigated whether Fgfr2 acts specifically in peri-Wolffian duct stroma (ST) to regulate UB induction and development of VUR and the mechanisms of Fgfr2 activity.
Methods: We conditionally deleted Fgfr2 in ST (Fgfr2(ST-/-)) using Tbx18cre mice. To look for ureteric bud induction defects in young embryos, we assessed length and apoptosis of common nephric ducts (CNDs). We performed 3D reconstructions and histological analyses of urinary tracts of embryos and postnatal mice and cystograms in postnatal mice to test for VUR. We performed in situ hybridization and real-time PCR in young embryos to determine mechanisms underlying UB induction defects.
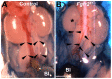
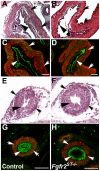
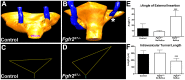
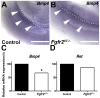
Results: We confirmed that Fgfr2 is expressed in ST and that Fgfr2 was efficiently deleted in this tissue in Fgfr2(ST-/-) mice at embryonic day (E) 10.5. E11.5 Fgfr2(ST-/-) mice had randomized UB induction sites with approximately 1/3 arising too high and 1/3 too low from the Wolffian duct; however, apoptosis was unaltered in E12.5 mutant CNDs. While ureters were histologically normal, E15.5 Fgfr2(ST-/-) mice exhibit improper ureteral insertion sites into the bladder, consistent with the ureteric induction defects. While ureter and bladder histology appeared normal, postnatal day (P) 1 mutants had high rates of VUR versus controls (75% versus 3%, p = 0.001) and occasionally other defects including renal hypoplasia and duplex systems. P1 mutant mice also had improper ureteral bladder insertion sites and shortened intravesicular tunnel lengths that correlated with VUR. E10.5 Fgfr2(ST-/-) mice had decreases in Bmp4 mRNA in stromal tissues, suggesting a mechanism underlying the ureteric induction and VUR phenotypes.
Conclusion: Mutations in FGFR2 could possibly cause VUR in humans.
Conflict of interest statement
Figures




Comment in
-
Findings of Research Misconduct.NIH Guide Grants Contracts (Bethesda). 2016 May 20:NOT-OD-16-090. NIH Guide Grants Contracts (Bethesda). 2016. PMID: 27220120 Free PMC article. No abstract available.
-
Findings of Research Misconduct.Fed Regist. 2016 May 11;81(91):29278. Fed Regist. 2016. PMID: 27737276 Free PMC article. No abstract available.
References
-
- Michos O, Goncalves A, Lopez-Rios J, Tiecke E, Naillat F, et al. (2007) Reduction of BMP4 activity by gremlin 1 enables ureteric bud outgrowth and GDNF/WNT11 feedback signalling during kidney branching morphogenesis. Development 134: 2397–2405. - PubMed
-
- Mackie GG, Awang H, Stephens FD (1975) The ureteric orifice: the embryologic key to radiologic status of duplex kidneys. J Pediatr Surg 10: 473–481. - PubMed
-
- Mackie GG, Stephens FD (1975) Duplex kidneys: a correlation of renal dysplasia with position of the ureteral orifice. J Urol 114: 274–280. - PubMed
-
- Murawski IJ, Gupta IR (2006) Vesicoureteric reflux and renal malformations: a developmental problem. Clin Genet 69: 105–117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

