Hepatocyte growth factor activator inhibitor-1 is induced by bone morphogenetic proteins and regulates proliferation and cell fate of neural progenitor cells
- PMID: 23409135
- PMCID: PMC3567048
- DOI: 10.1371/journal.pone.0056117
Hepatocyte growth factor activator inhibitor-1 is induced by bone morphogenetic proteins and regulates proliferation and cell fate of neural progenitor cells
Abstract
Background: Neural progenitor cells (NPCs) in the developing neuroepithelium are regulated by intrinsic and extrinsic factors. There is evidence that NPCs form a self-supporting niche for cell maintenance and proliferation. However, molecular interactions and cell-cell contacts and the microenvironment within the neuroepithelium are largely unknown. We hypothesized that cellular proteases especially those associated with the cell surface of NPCs play a role in regulation of progenitor cells in the brain.
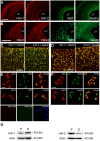
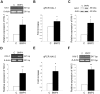
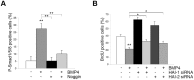
Methodology/principal findings: In this work, we show that NPCs, isolated from striatal anlage of developing rat brain, express hepatocyte growth factor activator inhibitor-1 and -2 (HAI-1 and HAI-2) that are cell surface-linked serine protease inhibitors. In addition, radial glia cells derived from mouse embryonic stem cells also express HAI-1 and HAI-2. To study the functional significance of HAI-1 and HAI-2 in progenitor cells, we modulated their levels using expression plasmids or silencing RNA (siRNA) transfected into the NPCs. Data showed that overexpression of HAI-1 or HAI-2 decreased cell proliferation of cultured NPCs, whilst their siRNAs had opposite effects. HAI-1 also influenced NPC differentiation by increasing the number of glial fibrillary acidic protein (GFAP) expressing cells in the culture. Expression of HAI-1 in vivo decreased cell proliferation in developing neuroepithelium in E15 old animals and promoted astrocyte cell differentiation in neonatal animals. Studying the regulation of HAI-1, we observed that Bone morphogenetic protein-2 (BMP-2) and BMP-4 increased HAI-1 levels in the NPCs. Experiments using HAI-1-siRNA showed that these BMPs act on the NPCs partly in a HAI-1-dependent manner.
Conclusions: This study shows that the cell-surface serine protease inhibitors, HAI-1 and HAI-2 influence proliferation and cell fate of NPCs and their expression levels are linked to BMP signaling. Modulation of the levels and actions of HAI-1 in NPCs may be of a potential value in stem cell therapies in various brain diseases.
Conflict of interest statement
Figures





Similar articles
-
mGluR3 promotes proliferation of human embryonic cortical neural progenitor cells by activating ERK1/2 and JNK2 signaling pathway in vitro.Cell Mol Biol (Noisy-le-grand). 2014 Sep 6;60(2):42-9. Cell Mol Biol (Noisy-le-grand). 2014. PMID: 25198581
-
Activation of mGluR4 promotes proliferation of rat neural progenitor cells while mediating activation of ERK1/2 signaling pathway.Cell Mol Biol (Noisy-le-grand). 2013 Jan 28;59 Suppl:OL1809-17. Cell Mol Biol (Noisy-le-grand). 2013. PMID: 23374450
-
Methylprednisolone inhibits the proliferation and affects the differentiation of rat spinal cord-derived neural progenitor cells cultured in low oxygen conditions by inhibiting HIF-1α and Hes1 in vitro.Int J Mol Med. 2014 Sep;34(3):788-95. doi: 10.3892/ijmm.2014.1835. Epub 2014 Jul 3. Int J Mol Med. 2014. PMID: 24992925
-
Bone morphogenetic proteins.Growth Factors. 2004 Dec;22(4):233-41. doi: 10.1080/08977190412331279890. Growth Factors. 2004. PMID: 15621726 Review.
-
N-cadherin-based adherens junction regulates the maintenance, proliferation, and differentiation of neural progenitor cells during development.Cell Adh Migr. 2015;9(3):183-92. doi: 10.1080/19336918.2015.1005466. Epub 2015 Apr 14. Cell Adh Migr. 2015. PMID: 25869655 Free PMC article. Review.
Cited by
-
Clinical Scale MSC-Derived Extracellular Vesicles Enhance Poststroke Neuroplasticity in Rodents and Non-Human Primates.J Extracell Vesicles. 2025 Jun;14(6):e70110. doi: 10.1002/jev2.70110. J Extracell Vesicles. 2025. PMID: 40545933 Free PMC article.
-
The crystal structure of a multidomain protease inhibitor (HAI-1) reveals the mechanism of its auto-inhibition.J Biol Chem. 2017 May 19;292(20):8412-8423. doi: 10.1074/jbc.M117.779256. Epub 2017 Mar 27. J Biol Chem. 2017. PMID: 28348076 Free PMC article.
-
HAI-1 is an independent predictor of lung cancer mortality and is required for M1 macrophage polarization.PLoS One. 2021 Jun 29;16(6):e0252197. doi: 10.1371/journal.pone.0252197. eCollection 2021. PLoS One. 2021. PMID: 34185790 Free PMC article.
-
Recombinant hepatocyte growth factor activator inhibitor 1: expression in Drosophila S2 cells, purification and crystallization.Acta Crystallogr F Struct Biol Commun. 2017 Jan 1;73(Pt 1):45-50. doi: 10.1107/S2053230X16020082. Epub 2017 Jan 1. Acta Crystallogr F Struct Biol Commun. 2017. PMID: 28045393 Free PMC article.
-
Immunophenotyping of Live Human Pluripotent Stem Cells by Flow Cytometry.Methods Mol Biol. 2018;1722:127-149. doi: 10.1007/978-1-4939-7553-2_9. Methods Mol Biol. 2018. PMID: 29264803 Free PMC article.
References
-
- Temple S (2001) The development of neural stem cells. Nature 414: 112–117. - PubMed
-
- Kataoka H, Suganuma T, Shimomura T, Itoh H, Kitamura N, et al. (1999) Distribution of hepatocyte growth factor activator inhibitor type 1 (HAI-1) in human tissues. Cellular surface localization of HAI-1 in simple columnar epithelium and its modulated expression in injured and regenerative tissues. J Histochem Cytochem 47: 673–682. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

