Overexpression of a fungal β-mannanase from Bispora sp. MEY-1 in maize seeds and enzyme characterization
- PMID: 23409143
- PMCID: PMC3569411
- DOI: 10.1371/journal.pone.0056146
Overexpression of a fungal β-mannanase from Bispora sp. MEY-1 in maize seeds and enzyme characterization
Abstract
Background: Mannans and heteromannans are widespread in plants cell walls and are well-known as anti-nutritional factors in animal feed. To remove these factors, it is common practice to incorporate endo-β-mannanase into feed for efficient nutrition absorption. The objective of this study was to overexpress a β-mannanase gene directly in maize, the main ingredient of animal feed, to simplify the process of feed production.
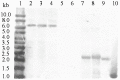
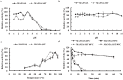
Methodology/principal findings: The man5A gene encoding an excellent β-mannanase from acidophilic Bispora sp. MEY-1 was selected for heterologous overexpression. Expression of the modified gene (man5As) was driven by the embryo-specific promoter ZM-leg1A, and the transgene was transferred to three generations by backcrossing with commercial inbred Zheng58. Its exogenous integration into the maize embryonic genome and tissue specific expression in seeds were confirmed by PCR and Southern blot and Western blot analysis, respectively. Transgenic plants at BC3 generation showed agronomic traits statistically similar to Zheng58 except for less plant height (154.0 cm vs 158.3 cm). The expression level of MAN5AS reached up to 26,860 units per kilogram of maize seeds. Compared with its counterpart produced in Pichia pastoris, seed-derived MAN5AS had higher temperature optimum (90°C), and remained more β-mannanase activities after pelleting at 80°C, 100°C or 120°C.
Conclusion/significance: This study shows the genetically stable overexpression of a fungal β-mannanase in maize and offers an effective and economic approach for transgene containment in maize for direct utilization without any purification or supplementation procedures.
Conflict of interest statement
Figures



Similar articles
-
Production of a Highly Protease-Resistant Fungal α-Galactosidase in Transgenic Maize Seeds for Simplified Feed Processing.PLoS One. 2015 Jun 8;10(6):e0129294. doi: 10.1371/journal.pone.0129294. eCollection 2015. PLoS One. 2015. PMID: 26053048 Free PMC article.
-
Overexpression of an acidic endo-β-1,3-1,4-glucanase in transgenic maize seed for direct utilization in animal feed.PLoS One. 2013 Dec 31;8(12):e81993. doi: 10.1371/journal.pone.0081993. eCollection 2013. PLoS One. 2013. PMID: 24391711 Free PMC article.
-
A novel highly acidic beta-mannanase from the acidophilic fungus Bispora sp. MEY-1: gene cloning and overexpression in Pichia pastoris.Appl Microbiol Biotechnol. 2009 Mar;82(3):453-61. doi: 10.1007/s00253-008-1766-x. Epub 2008 Nov 8. Appl Microbiol Biotechnol. 2009. PMID: 18998121
-
An alpha-galactosidase from an acidophilic Bispora sp. MEY-1 strain acts synergistically with beta-mannanase.Bioresour Technol. 2010 Nov;101(21):8376-82. doi: 10.1016/j.biortech.2010.06.045. Epub 2010 Jun 29. Bioresour Technol. 2010. PMID: 20591661
-
Seed-specific expression of the lysine-rich protein gene sb401 significantly increases both lysine and total protein content in maize seeds.Food Nutr Bull. 2005 Dec;26(4):427-31. Food Nutr Bull. 2005. PMID: 16465991 Review.
Cited by
-
Production of a Highly Protease-Resistant Fungal α-Galactosidase in Transgenic Maize Seeds for Simplified Feed Processing.PLoS One. 2015 Jun 8;10(6):e0129294. doi: 10.1371/journal.pone.0129294. eCollection 2015. PLoS One. 2015. PMID: 26053048 Free PMC article.
-
Fungi, a neglected component of acidophilic biofilms: do they have a potential for biotechnology?Extremophiles. 2019 May;23(3):267-275. doi: 10.1007/s00792-019-01085-9. Epub 2019 Mar 6. Extremophiles. 2019. PMID: 30840146 Review.
-
Root Transcriptome Analysis of Wild Peanut Reveals Candidate Genes for Nematode Resistance.PLoS One. 2015 Oct 21;10(10):e0140937. doi: 10.1371/journal.pone.0140937. eCollection 2015. PLoS One. 2015. PMID: 26488731 Free PMC article.
-
Strategies for the production of cell wall-deconstructing enzymes in lignocellulosic biomass and their utilization for biofuel production.Plant Biotechnol J. 2016 Jun;14(6):1329-44. doi: 10.1111/pbi.12505. Epub 2015 Dec 2. Plant Biotechnol J. 2016. PMID: 26627868 Free PMC article. Review.
-
Three Parts of the Plant Genome: On the Way to Success in the Production of Recombinant Proteins.Plants (Basel). 2022 Dec 21;12(1):38. doi: 10.3390/plants12010038. Plants (Basel). 2022. PMID: 36616166 Free PMC article. Review.
References
-
- McCleary BV (1988) β-Mannanase. Method Enzymol 160: 596–610.
-
- Moreira LRS, Filho EXF (2008) An overview of mannan structure and mannan- degrading enzyme systems. Appl Microbiol Biotechnol 79: 165–178. - PubMed
-
- Petkowicz CLO, Reicher F, Chanzy H, Taravel FR, Vuong R (2001) Linear mannan in the endosperm of Schizolobium amazonicum . Carbohyd Polym 44: 107–112.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

