Spatial distribution and receptor specificity of zebrafish Kit system--evidence for a Kit-mediated bi-directional communication system in the preovulatory ovarian follicle
- PMID: 23409152
- PMCID: PMC3568072
- DOI: 10.1371/journal.pone.0056192
Spatial distribution and receptor specificity of zebrafish Kit system--evidence for a Kit-mediated bi-directional communication system in the preovulatory ovarian follicle
Abstract
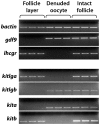
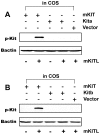
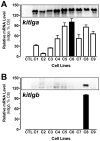
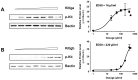
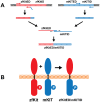
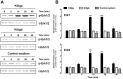
Consisting of Kit ligand and receptor Kit, the Kit system is involved in regulating many ovarian functions such as follicle activation, granulosa cell proliferation, and oocyte growth and maturation. In mammals, Kit ligand is derived from the granulosa cells and Kit receptor is expressed in the oocyte and theca cells. In the zebrafish, the Kit system contains two ligands (Kitlga and Kitlgb) and two receptors (Kita and Kitb). Interestingly, Kitlga and Kitb are localized in the somatic follicle cells, but Kitlgb and Kita are expressed in the oocyte. Using recombinant zebrafish Kitlga and Kitlgb, we demonstrated that Kitlga preferentially activated Kita whereas Kitlgb specifically activated Kitb by Western analysis for receptor phosphorylation. In support of this, Kitlgb triggered a stronger and longer MAPK phosphorylation in follicle cells than Kitlga, whereas Kitlga but not Kitlgb activated MAPK in the denuded oocytes, in agreement with the distribution of Kita and Kitb in the follicle and their specificity for Kitlga and Kitlgb. Further analysis of the interaction between Kit ligands and receptors by homology modeling showed that Kitlga-Kita and Kitlgb-Kitb both have more stable electrostatic interaction than Kitlgb-Kita or Kitlga-Kitb. A functional study of Kit involvement in final oocyte maturation showed that Kitlga and Kitlgb both suppressed the spontaneous maturation significantly; in contrast, Kitlgb but not Kitlga significantly promoted 17α, 20β-dihydroxy-4-pregnen-3-one (DHP) -induced oocyte maturation. Our results provided strong evidence for a Kit-mediated bi-directional communication system in the zebrafish ovarian follicle, which could be part of the complex interplay between the oocyte and the follicle cells in the development of follicles.
Conflict of interest statement
Figures








References
-
- Copeland NG, Gilbert DJ, Cho BC, Donovan PJ, Jenkins NA, et al. (1990) Mast cell growth factor maps near the steel locus on mouse chromosome 10 and is deleted in a number of steel alleles. Cell 63: 175–183. - PubMed
-
- Huang E, Nocka K, Beier DR, Chu TY, Buck J, et al. (1990) The hematopoietic growth factor KL is encoded by the Sl locus and is the ligand of the c-kit receptor, the gene product of the W locus. Cell 63: 225–233. - PubMed
-
- Matsui Y, Zsebo KM, Hogan BL (1990) Embryonic expression of a haematopoietic growth factor encoded by the Sl locus and the ligand for c-kit. Nature 347: 667–669. - PubMed
-
- Chabot B, Stephenson DA, Chapman VM, Besmer P, Bernstein A (1988) The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature 335: 88–89. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

