A parent-of-origin effect determines the susceptibility of a non-informative F1 population to Trypanosoma cruzi infection in vivo
- PMID: 23409175
- PMCID: PMC3569416
- DOI: 10.1371/journal.pone.0056347
A parent-of-origin effect determines the susceptibility of a non-informative F1 population to Trypanosoma cruzi infection in vivo
Abstract
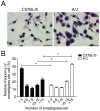
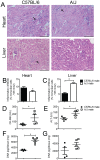
The development of Chagas disease is determined by a complex interaction between the genetic traits of both the protozoan parasite, T. cruzi, and the infected host. This process is regulated by multiple genes that control different aspects of the host-parasite interaction. While determination of the relevant genes in humans is extremely difficult, it is feasible to use inbred mouse strains to determine the genes and loci responsible for host resistance to infection. In this study, we investigated the susceptibility of several inbred mouse strains to infection with the highly virulent Y strain of T. cruzi and found a considerable difference in susceptibility between A/J and C57BL/6 mice. We explored the differences between these two mouse strains and found that the A/J strain presented higher mortality, exacerbated and uncontrolled parasitemia and distinct histopathology in the target organs, which were associated with a higher parasite burden and more extensive tissue lesions. We then employed a genetic approach to assess the pattern of inheritance of the resistance phenotype in an F1 population and detected a strong parent-of-origin effect determining the susceptibility of the F1 male mice. This effect is unlikely to result from imprinted genes because the inheritance of this susceptibility was affected by the direction of the parental crossing. Collectively, our genetic approach of using the F1 population suggests that genes contained in the murine chromosome X contribute to the natural resistance against T. cruzi infection. Future linkage studies may reveal the locus and genes participating on the host resistance process reported herein.
Conflict of interest statement
Figures




Similar articles
-
Genes from Chagas susceptibility loci that are differentially expressed in T. cruzi-resistant mice are candidates accounting for impaired immunity.PLoS One. 2006 Dec 20;1(1):e57. doi: 10.1371/journal.pone.0000057. PLoS One. 2006. PMID: 17183687 Free PMC article.
-
Studies of Trypanosoma cruzi clones in inbred mice. II. Course of infection of C57BL/6 mice with single-cell-isolated stocks.Am J Trop Med Hyg. 1984 Mar;33(2):236-8. doi: 10.4269/ajtmh.1984.33.236. Am J Trop Med Hyg. 1984. PMID: 6424485
-
Trypanosoma cruzi: role of host genetic background in the differential tissue distribution of parasite clonal populations.Exp Parasitol. 2002 Apr;100(4):269-75. doi: 10.1016/s0014-4894(02)00024-3. Exp Parasitol. 2002. PMID: 12128054
-
Trypanosoma cruzi genetic diversity: Something new for something known about Chagas disease manifestations, serodiagnosis and drug sensitivity.Acta Trop. 2018 Aug;184:38-52. doi: 10.1016/j.actatropica.2017.09.017. Epub 2017 Sep 21. Acta Trop. 2018. PMID: 28941731 Review.
-
Trypanosoma cruzi infection by oral route: how the interplay between parasite and host components modulates infectivity.Parasitol Int. 2008 Jun;57(2):105-9. doi: 10.1016/j.parint.2007.12.008. Epub 2007 Dec 23. Parasitol Int. 2008. PMID: 18234547 Review.
Cited by
-
Relevance of Trypanothione Reductase Inhibitors on Trypanosoma cruzi Infection: A Systematic Review, Meta-Analysis, and In Silico Integrated Approach.Oxid Med Cell Longev. 2018 Oct 24;2018:8676578. doi: 10.1155/2018/8676578. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 30473742 Free PMC article.
-
The use of a heterogeneously controlled mouse population reveals a significant correlation of acute phase parasitemia with mortality in Chagas disease.PLoS One. 2014 Mar 20;9(3):e91640. doi: 10.1371/journal.pone.0091640. eCollection 2014. PLoS One. 2014. PMID: 24651711 Free PMC article.
-
T-cell receptor variable region usage in Chagas disease: A systematic review of experimental and human studies.PLoS Negl Trop Dis. 2022 Sep 15;16(9):e0010546. doi: 10.1371/journal.pntd.0010546. eCollection 2022 Sep. PLoS Negl Trop Dis. 2022. PMID: 36107855 Free PMC article.
-
Host Genetics Background Influence in the Intragastric Trypanosoma cruzi Infection.Front Immunol. 2020 Nov 24;11:566476. doi: 10.3389/fimmu.2020.566476. eCollection 2020. Front Immunol. 2020. PMID: 33329529 Free PMC article.
-
Trypanosoma cruzi infection in genetically selected mouse lines: genetic linkage with quantitative trait locus controlling antibody response.Mediators Inflamm. 2014;2014:952857. doi: 10.1155/2014/952857. Epub 2014 Aug 13. Mediators Inflamm. 2014. PMID: 25197170 Free PMC article.
References
-
- Chapman SJ, Hill AV (2012) Human genetic susceptibility to infectious disease. Nature Reviews Genetics 13: 175–188. - PubMed
-
- Villasenor-Cardoso MI, Ortega E (2011) Polymorphisms of innate immunity receptors in infection by parasites. Parasite Immunology 33: 643–653. - PubMed
-
- WHO (2007) Report of the scientific working group on Chagas disease.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

