Non-image-forming light driven functions are preserved in a mouse model of autosomal dominant optic atrophy
- PMID: 23409176
- PMCID: PMC3569441
- DOI: 10.1371/journal.pone.0056350
Non-image-forming light driven functions are preserved in a mouse model of autosomal dominant optic atrophy
Abstract
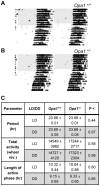
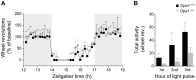
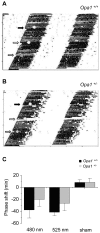
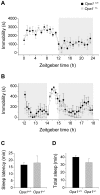
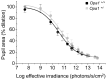
Autosomal dominant optic atrophy (ADOA) is a slowly progressive optic neuropathy that has been associated with mutations of the OPA1 gene. In patients, the disease primarily affects the retinal ganglion cells (RGCs) and causes optic nerve atrophy and visual loss. A subset of RGCs are intrinsically photosensitive, express the photopigment melanopsin and drive non-image-forming (NIF) visual functions including light driven circadian and sleep behaviours and the pupil light reflex. Given the RGC pathology in ADOA, disruption of NIF functions might be predicted. Interestingly in ADOA patients the pupil light reflex was preserved, although NIF behavioural outputs were not examined. The B6; C3-Opa1(Q285STOP) mouse model of ADOA displays optic nerve abnormalities, RGC dendropathy and functional visual disruption. We performed a comprehensive assessment of light driven NIF functions in this mouse model using wheel running activity monitoring, videotracking and pupillometry. Opa1 mutant mice entrained their activity rhythm to the external light/dark cycle, suppressed their activity in response to acute light exposure at night, generated circadian phase shift responses to 480 nm and 525 nm pulses, demonstrated immobility-defined sleep induction following exposure to a brief light pulse at night and exhibited an intensity dependent pupil light reflex. There were no significant differences in any parameter tested relative to wildtype littermate controls. Furthermore, there was no significant difference in the number of melanopsin-expressing RGCs, cell morphology or melanopsin transcript levels between genotypes. Taken together, these findings suggest the preservation of NIF functions in Opa1 mutants. The results provide support to growing evidence that the melanopsin-expressing RGCs are protected in mitochondrial optic neuropathies.
Conflict of interest statement
Figures

References
-
- Kjer P (1959) Infantile optic atrophy with dominant mode of inheritance: a clinical and genetic study of 19 Danish families. Acta Ophthalmol Suppl 164: 1–147. - PubMed
-
- Kline LB, Glaser JS (1979) Dominant optic atrophy. The clinical profile. Arch Ophthalmol 97: 1680–1686. - PubMed
-
- Votruba M, Fitzke FW, Holder GE, Carter A, Bhattacharya SS, et al. (1998) Clinical features in affected individuals from 21 pedigrees with dominant optic atrophy. Arch Ophthalmol 116: 351–358. - PubMed
-
- Johnston PB, Gaster RN, Smith VC, Tripathi RC (1979) A clinicopathologic study of autosomal dominant optic atrophy. Am J Ophthalmol 88: 868–875. - PubMed
-
- Kjer P, Jensen OA, Klinken L (1983) Histopathology of eye, optic nerve and brain in a case of dominant optic atrophy. Acta Ophthalmol (Copenh) 61: 300–312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

