In vitro characterization of circulating endothelial progenitor cells isolated from patients with acute coronary syndrome
- PMID: 23409178
- PMCID: PMC3569417
- DOI: 10.1371/journal.pone.0056377
In vitro characterization of circulating endothelial progenitor cells isolated from patients with acute coronary syndrome
Abstract
Background: The current understanding of the functional characteristics of circulating endothelial progenitor cells (EPC) is limited, especially in patients affected by cardiovascular diseases. In this study, we have analyzed the in vitro clonogenic capacity of circulating EPC, also known as endothelial colony-forming cells (ECFC), in patients with acute coronary syndrome (ACS), in comparison to the colony forming unit-endothelial-like cells (CFU-EC) of hematopoietic/monocytic origin.
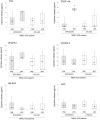
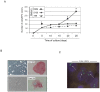
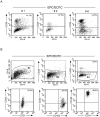
Methodology/principal findings: By culturing peripheral blood mononuclear cells (PBMC) of patients with ACS (n = 70), CFU-EC were frequently isolated (from 77% of ACS patients), while EPC/ECFC were obtained only in a small subset (13%) of PBMC samples, all harvested between 7-14 days after the acute cardiovascular event. Notably, ex-vivo generation of EPC/ECFC was correlated to a higher in vitro release of PDGF-AA by the corresponding ACS patient PBMC. By using specific endothelial culture media, EPC/ECFC displayed in vitro expansion capacity, allowing the phenotypic and functional characterization of the cells. Indeed, after expansion, EPC/ECFC exhibited a normal diploid chromosomal setting by FISH analysis and an immunophenotype characterized by: i) uniform positivity for the expression of CD105, CD31, CD146 and Factor VIII, i) variable expression of the CD34, CD106 and CD184 markers, and iii) negativity for CD45, CD90, CD117 and CD133. Of interest, in single-cell replanting assays EPC/ECFC exhibited clonogenic expansion capacity, forming secondary colonies characterized by variable proliferation capacities.
Conclusion/significance: Our data indicate that a careful characterization of true EPC is needed in order to design future studies in the clinical autologous setting of patients with ACS.
Conflict of interest statement
Figures


References
-
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, et al. (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275: 964–967. - PubMed
-
- Schatteman GC, Dunnwald M, Jiao C (2006) Biology of bone marrow derived endothelial precursors. Am J Physiol Heart Circ Physiol 292: 1–18. - PubMed
-
- Shi Q, Rafii S, Wu MHD, Wijelath ES, Cong Y, et al. (1998) Evidence for circulating bone marrow-derived endothelial cells. Blood 92: 362–367. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

