Effect of delayed peripheral nerve repair on nerve regeneration, Schwann cell function and target muscle recovery
- PMID: 23409189
- PMCID: PMC3567071
- DOI: 10.1371/journal.pone.0056484
Effect of delayed peripheral nerve repair on nerve regeneration, Schwann cell function and target muscle recovery
Abstract
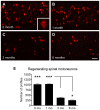
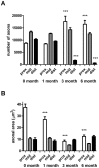
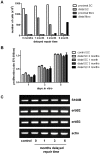
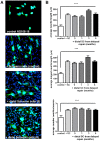
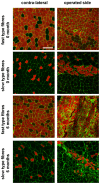
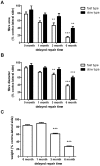
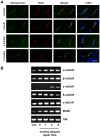
Despite advances in surgical techniques for peripheral nerve repair, functional restitution remains incomplete. The timing of surgery is one factor influencing the extent of recovery but it is not yet clearly defined how long a delay may be tolerated before repair becomes futile. In this study, rats underwent sciatic nerve transection before immediate (0) or 1, 3, or 6 months delayed repair with a nerve graft. Regeneration of spinal motoneurons, 13 weeks after nerve repair, was assessed using retrograde labeling. Nerve tissue was also collected from the proximal and distal stumps and from the nerve graft, together with the medial gastrocnemius (MG) muscles. A dramatic decline in the number of regenerating motoneurons and myelinated axons in the distal nerve stump was observed in the 3- and 6-months delayed groups. After 3 months delay, the axonal number in the proximal stump increased 2-3 folds, accompanied by a smaller axonal area. RT-PCR of distal nerve segments revealed a decline in Schwann cells (SC) markers, most notably in the 3 and 6 month delayed repair samples. There was also a progressive increase in fibrosis and proteoglycan scar markers in the distal nerve with increased delayed repair time. The yield of SC isolated from the distal nerve segments progressively fell with increased delay in repair time but cultured SC from all groups proliferated at similar rates. MG muscle at 3- and 6-months delay repair showed a significant decline in weight (61% and 27% compared with contra-lateral side). Muscle fiber atrophy and changes to neuromuscular junctions were observed with increased delayed repair time suggestive of progressively impaired reinnervation. This study demonstrates that one of the main limiting factors for nerve regeneration after delayed repair is the distal stump. The critical time point after which the outcome of regeneration becomes too poor appears to be 3-months.
Conflict of interest statement
Figures


References
-
- Wiberg M, Terenghi G (2003) Will it be possible to produce peripheral nerves? Surg Technol Int 11: 303–310. - PubMed
-
- Lad SP, Nathan JK, Schubert RD, Boakye M (2010) Trends in median, ulnar, radial, and brachioplexus nerve injuries in the United States. Neurosurgery 66: 953–960. - PubMed
-
- Vejsada R, Sagot Y, Kato AC (1995) Quantitative comparison of the transient rescue effects of neurotrophic factors on axotomized motoneurons in vivo. Eur J Neurosci 7: 108–115. - PubMed
-
- Novikova L, Novikov L, Kellerth JO (1997) Persistent neuronal labeling by retrograde fluorescent tracers: a comparison between Fast Blue, Fluoro-Gold and various dextran conjugates. J Neurosci Methods 74: 9–15. - PubMed
-
- Welin D, Novikova LN, Wiberg M, Kellerth JO, Novikov LN (2008) Survival and regeneration of cutaneous and muscular afferent neurons after peripheral nerve injury in adult rats. Exp Brain Res 186: 315–323. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

