Head and neck cancer cells and xenografts are very sensitive to palytoxin: decrease of c-jun n-terminale kinase-3 expression enhances palytoxin toxicity
- PMID: 23409748
- PMCID: PMC3585753
- DOI: 10.1186/1476-4598-12-12
Head and neck cancer cells and xenografts are very sensitive to palytoxin: decrease of c-jun n-terminale kinase-3 expression enhances palytoxin toxicity
Abstract
Objectives: Palytoxin (PTX), a marine toxin isolated from the Cnidaria (zooanthid) Palythoa caribaeorum is one of the most potent non-protein substances known. It is a very complex molecule that presents both lipophilic and hydrophilic areas. The effect of PTX was investigated in a series of experiments conducted in head and neck squamous cell carcinoma (HNSCC) cell lines and xenografts.
Materials and methods: Cell viability, and gene expression of the sodium/potassium-transporting ATPase subumit alpha1 (ATP1AL1) and GAPDH were analyzed in HNSCC cells and normal epithelial cells after treatment with PTX using cytotoxicity-, clonogenic-, and enzyme inhibitor assays as well as RT-PCR and Northern Blotting. For xenograft experiments severe combined immunodeficient (SCID) mice were used to analyze tumor regression. The data were statistically analyzed using One-Way Annova (SPSS vs20).
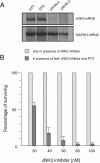
Results: Significant toxic effects were observed in tumor cells treated with PTX (LD50 of 1.5 to 3.5 ng/ml) in contrast to normal cells. In tumor cells PTX affected both the release of LDH and the expression of the sodium/potassium-transporting ATPase subunit alpha1 gene suggesting loss of cellular integrity, primarily of the plasma membrane. Furthermore, strong repression of the c-Jun N-terminal kinase 3 (JNK3) mRNA expression was found in carcinoma cells which correlated with enhanced toxicity of PTX suggesting an essential role of the mitogen activated protein kinase (MAPK)/JNK signalling cascades pathway in the mechanisms of HNSCC cell resistance to PTX. In mice inoculated with carcinoma cells, injections of PTX into the xenografted tumors resulted within 24 days in extensive tumor destruction in 75% of the treated animals (LD50 of 68 ng/kg to 83 ng/kg) while no tumor regression occurred in control animals.
Conclusions: These results clearly provide evidence that PTX possesses preferential toxicity for head and neck carcinoma cells and therefore it is worth further studying its impact which may extend our knowledge of the biology of head and neck cancer.
Figures


References
-
- Kan Y, Uemura D, Hirata Y, Ishiguro M, Iwashita T. Complete NMR signal assignment of palytoxin and N-acetylpalytoxin. Tetrahedron Let. 2001;42(18):3197–3202. doi: 10.1016/S0040-4039(01)00407-5. - DOI
-
- Bignami GS, Senter PD, Grothaus PG, Fischer KJ, Humphreys T, Wallace PM. N-(4’-hydroxyphenylacetyl)palytoxin: a palytoxin prodrug that can be activated by a monoclonal antibody-penicillin g amidase conjugat. Cancer Res. 1992;52(20):5759–5764. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

