Sodium vanadate combined with L-ascorbic acid delays disease progression, enhances motor performance, and ameliorates muscle atrophy and weakness in mice with spinal muscular atrophy
- PMID: 23409868
- PMCID: PMC3682891
- DOI: 10.1186/1741-7015-11-38
Sodium vanadate combined with L-ascorbic acid delays disease progression, enhances motor performance, and ameliorates muscle atrophy and weakness in mice with spinal muscular atrophy
Abstract
Background: Proximal spinal muscular atrophy (SMA), a neurodegenerative disorder that causes infant mortality, has no effective treatment. Sodium vanadate has shown potential for the treatment of SMA; however, vanadate-induced toxicity in vivo remains an obstacle for its clinical application. We evaluated the therapeutic potential of sodium vanadate combined with a vanadium detoxification agent, L-ascorbic acid, in a SMA mouse model.
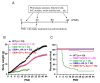
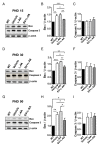
Methods: Sodium vanadate (200 μM), L-ascorbic acid (400 μM), or sodium vanadate combined with L-ascorbic acid (combined treatment) were applied to motor neuron-like NSC34 cells and fibroblasts derived from a healthy donor and a type II SMA patient to evaluate the cellular viability and the efficacy of each treatment in vitro. For the in vivo studies, sodium vanadate (20 mg/kg once daily) and L-ascorbic acid (40 mg/kg once daily) alone or in combination were orally administered daily on postnatal days 1 to 30. Motor performance, pathological studies, and the effects of each treatment (vehicle, L-ascorbic acid, sodium vanadate, and combined treatment) were assessed and compared on postnatal days (PNDs) 30 and 90. The Kaplan-Meier method was used to evaluate the survival rate, with P < 0.05 indicating significance. For other studies, one-way analysis of variance (ANOVA) and Student's t test for paired variables were used to measure significant differences (P < 0.05) between values.
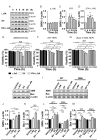
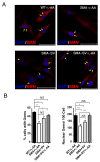
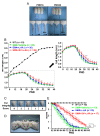
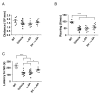
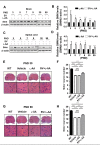
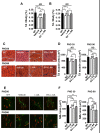
Results: Combined treatment protected cells against vanadate-induced cell death with decreasing B cell lymphoma 2-associated X protein (Bax) levels. A month of combined treatment in mice with late-onset SMA beginning on postnatal day 1 delayed disease progression, improved motor performance in adulthood, enhanced survival motor neuron (SMN) levels and motor neuron numbers, reduced muscle atrophy, and decreased Bax levels in the spinal cord. Most importantly, combined treatment preserved hepatic and renal function and substantially decreased vanadium accumulation in these organs.
Conclusions: Combined treatment beginning at birth and continuing for 1 month conferred protection against neuromuscular damage in mice with milder types of SMA. Further, these mice exhibited enhanced motor performance in adulthood. Therefore, combined treatment could present a feasible treatment option for patients with late-onset SMA.
Figures
References
-
- Lefebvre S, Burglen L, Reboullet S, Clermont O, Burlet P, Viollet L, Benichou B, Cruaud C, Millasseau P, Zeviani M, Pasiler D, Frézal J, Cohen D, Weissenbach J, Munnich A, Melki J. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–165. doi: 10.1016/0092-8674(95)90460-3. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

