Advances in microfluidic materials, functions, integration, and applications
- PMID: 23410114
- PMCID: PMC3624029
- DOI: 10.1021/cr300337x
Advances in microfluidic materials, functions, integration, and applications
Figures
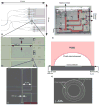




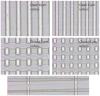












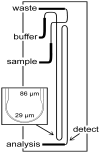



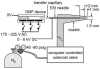

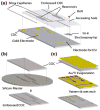

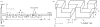


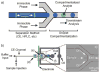
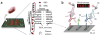




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

