Pathogenic mutation in VPS35 impairs its protection against MPP(+) cytotoxicity
- PMID: 23411763
- PMCID: PMC3572397
- DOI: 10.7150/ijbs.5617
Pathogenic mutation in VPS35 impairs its protection against MPP(+) cytotoxicity
Abstract
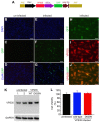
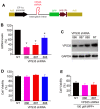
Parkinson's disease primarily results from progressive degeneration of dopaminergic neurons in the substantia nigra. Both neuronal toxicants and genetic factors are suggested to be involved in the disease pathogenesis. The mitochondrial toxicant 1-methyl-4-phenylpyridinium (MPP(+)) shows a highly selective toxicity to dopaminergic neurons. Recent studies indicate that mutation in the vacuolar protein sorting 35 (vps35) gene segregates with Parkinson's disease in some families, but how mutation in the vps35 gene causes dopaminergic cell death is not known. Here, we report that enhanced VPS35 expression protected dopaminergic cells against MPP(+) toxicity and that this neuroprotection was compromised by pathogenic mutation in the gene. A loss of neuroprotective functions contributes to the pathogenesis of VPS35 mutation in Parkinson's disease.
Keywords: 1-methyl-4-phenylpyridinium; MPP; Parkinson's disease; VPS35; vacuolar protein sorting 35.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


References
-
- Schulz JB. Mechanisms of neurodegeneration in idiopathic Parkinson's disease. Parkinsonism Relat Disord 13 Suppl. 2007;3:S306–308. - PubMed
-
- Xia XG, Schmidt N, Teismann P, Ferger B, Schulz JB. Dopamine mediates striatal malonate toxicity via dopamine transporter-dependent generation of reactive oxygen species and D2 but not D1 receptor activation. J Neurochem. 2001;79:63–70. - PubMed
-
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R. et al. Mu-tation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. - PubMed
-
- Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

