Effector-triggered versus pattern-triggered immunity: how animals sense pathogens
- PMID: 23411798
- PMCID: PMC4121468
- DOI: 10.1038/nri3398
Effector-triggered versus pattern-triggered immunity: how animals sense pathogens
Abstract
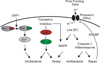
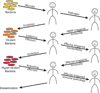
A fundamental question regarding any immune system is how it can discriminate between pathogens and non-pathogens. Here, we discuss how this discrimination can be mediated by a surveillance system distinct from pattern-recognition receptors that recognize conserved microbial patterns. It can be based instead on the ability of the host to sense perturbations in host cells induced by bacterial toxins or 'effectors' that are encoded by pathogenic microorganisms. Such 'effector-triggered immunity' was previously demonstrated mainly in plants, but recent data confirm that animals can also use this strategy.
Figures

References
-
- Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. - PubMed
-
- Medzhitov R. Approaching the asymptote: 20 years later. Immunity. 2009;30:766–775. - PubMed
-
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. - PubMed
-
- Medzhitov R. Innate immunity: quo vadis? Nat Immunol. 2010;11:551–553. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

