Organization of synthetic alphoid DNA array in human artificial chromosome (HAC) with a conditional centromere
- PMID: 23411994
- PMCID: PMC3568986
- DOI: 10.1021/sb3000436
Organization of synthetic alphoid DNA array in human artificial chromosome (HAC) with a conditional centromere
Abstract
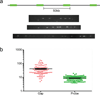
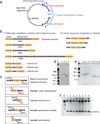
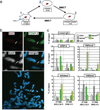
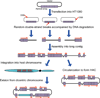
Human artificial chromosomes (HACs) represent a novel promising episomal system for functional genomics, gene therapy, and synthetic biology. HACs are engineered from natural and synthetic alphoid DNA arrays upon transfection into human cells. The use of HACs for gene expression studies requires the knowledge of their structural organization. However, none of the de novo HACs constructed so far has been physically mapped in detail. Recently we constructed a synthetic alphoid(tetO)-HAC that was successfully used for expression of full-length genes to correct genetic deficiencies in human cells. The HAC can be easily eliminated from cell populations by inactivation of its conditional kinetochore. This unique feature provides a control for phenotypic changes attributed to expression of HAC-encoded genes. This work describes organization of a megabase-size synthetic alphoid DNA array in the alphoid(tetO)-HAC that has been formed from a ~50 kb synthetic alphoid(tetO)-construct. Our analysis showed that this array represents a 1.1 Mb continuous sequence assembled from multiple copies of input DNA, a significant part of which was rearranged before assembling. The tandem and inverted alphoid DNA repeats in the HAC range in size from 25 to 150 kb. In addition, we demonstrated that the structure and functional domains of the HAC remains unchanged after several rounds of its transfer into different host cells. The knowledge of the alphoid(tetO)-HAC structure provides a tool to control HAC integrity during different manipulations. Our results also shed light on a mechanism for de novo HAC formation in human cells.
Figures


References
-
- Ren X, Tahimic CG, Katoh M, Kurimasa A, Inoue T, Oshimura M. Human artificial chromosome vectors meet stem cells: new prospects for gene delivery. Stem Cell Review. 2006;2:43–50. - PubMed
-
- Grimes BR, Monaco ZL. Artificial and engineered chromosomes: Developments and prospects for gene therapy. Chromosoma. 2005;114:230–241. - PubMed
-
- Basu J, Willard HF. Artificial and engineered chromosomes: Non-integrating vectors for gene therapy. Trends Mol. Med. 2005;11:251–258. - PubMed
-
- Monaco ZL, Moralli D. Progress in artificial chromosome technology. Biochem. Soc. Trans. 2006;34:324–327. - PubMed
-
- Macnab S, Whitehouse A. Progress and prospects: human artificial chromosomes. Gene Ther. 2009;16:1180–1188. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

