Canagliflozin lowers postprandial glucose and insulin by delaying intestinal glucose absorption in addition to increasing urinary glucose excretion: results of a randomized, placebo-controlled study
- PMID: 23412078
- PMCID: PMC3714520
- DOI: 10.2337/dc12-2391
Canagliflozin lowers postprandial glucose and insulin by delaying intestinal glucose absorption in addition to increasing urinary glucose excretion: results of a randomized, placebo-controlled study
Abstract
Objective: Canagliflozin, a sodium glucose cotransporter (SGLT) 2 inhibitor, is also a low-potency SGLT1 inhibitor. This study tested the hypothesis that intestinal canagliflozin levels postdose are sufficiently high to transiently inhibit intestinal SGLT1, thereby delaying intestinal glucose absorption.
Research design and methods: This two-period, crossover study evaluated effects of canagliflozin on intestinal glucose absorption in 20 healthy subjects using a dual-tracer method. Placebo or canagliflozin 300 mg was given 20 min before a 600-kcal mixed-meal tolerance test. Plasma glucose, (3)H-glucose, (14)C-glucose, and insulin were measured frequently for 6 h to calculate rates of appearance of oral glucose (RaO) in plasma, endogenous glucose production, and glucose disposal.
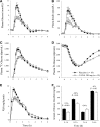
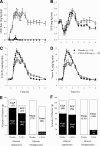
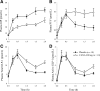
Results: Compared with placebo, canagliflozin treatment reduced postprandial plasma glucose and insulin excursions (incremental 0- to 2-h area under the curve [AUC0-2h] reductions of 35% and 43%, respectively; P < 0.001 for both), increased 0- to 6-h urinary glucose excretion (UGE0-6h, 18.2 ± 5.6 vs. <0.2 g; P < 0.001), and delayed RaO. Canagliflozin reduced AUC RaO by 31% over 0 to 1 h (geometric means, 264 vs. 381 mg/kg; P < 0.001) and by 20% over 0 to 2 h (576 vs. 723 mg/kg; P = 0.002). Over 2 to 6 h, canagliflozin increased RaO such that total AUC RaO over 0 to 6 h was <6% lower versus placebo (960 vs. 1,018 mg/kg; P = 0.003). A modest (∼10%) reduction in acetaminophen absorption was observed over the first 2 h, but this difference was not sufficient to explain the reduction in RaO. Total glucose disposal over 0 to 6 h was similar across groups.
Conclusions: Canagliflozin reduces postprandial plasma glucose and insulin by increasing UGE (via renal SGLT2 inhibition) and delaying RaO, likely due to intestinal SGLT1 inhibition.
Trial registration: ClinicalTrials.gov NCT01173549.
Figures
References
-
- Lee YJ, Lee YJ, Han HJ. Regulatory mechanisms of Na(+)/glucose cotransporters in renal proximal tubule cells. Kidney Int Suppl 2007;Aug:S27–S35 - PubMed
-
- Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev 2011;91:733–794 - PubMed
-
- Abdul-Ghani MA, Norton L, Defronzo RA. Role of sodium-glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr Rev 2011;32:515–531 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

