Delta-6-desaturase activity and arachidonic acid synthesis are increased in human breast cancer tissue
- PMID: 23414387
- PMCID: PMC7657148
- DOI: 10.1111/cas.12129
Delta-6-desaturase activity and arachidonic acid synthesis are increased in human breast cancer tissue
Abstract
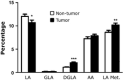
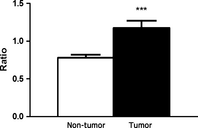
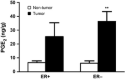
Omega-6 (n-6) arachidonic acid (AA) and its pro-inflammatory metabolites, including prostaglandin E2 (PGE(2)), are known to promote tumorigenesis. Delta-6 desaturase (D6D) is the rate-limiting enzyme for converting n-6 linoleic acid (LA) to AA. Our objective was to determine if AA synthesis, specifically D6D activity, and PGE(2) levels are increased in cancerous breast tissue, and whether these variables differ between estrogen receptor positive (ER+) and negative (ER-) breast cancers. Gas chromatography was performed on surgical breast tissue samples collected from 69 women with breast cancer. Fifty-four had ER+ breast cancer, and 15 had ER- breast cancer. Liquid chromatography-mass spectrometry was used to determine PGE(2) levels. Lipid analysis revealed higher levels of LA metabolites (C18:3 n-6, C20:3 n-6, and AA) in cancerous tissue than in adjacent noncancerous tissue (P < 0.01). The ratio of LA metabolites to LA, a measure of D6D activity, was increased in cancerous tissue, suggesting greater conversion of LA to AA (P < 0.001), and was higher in ER- than in ER+ patients, indicating genotype-related trends. Similarly, PGE(2) levels were increased in cancerous tissue, particularly in ER- patients. The results showed that the endogenous AA synthetic pathway, D6D activity, and PGE(2) levels are increased in breast tumors, particularly those of the ER- genotype. These findings suggest that the AA synthetic pathway and the D6D enzyme in particular may be involved in the pathogenesis of breast cancer. The development of drugs and nutritional interventions to alter this pathway may provide new strategies for breast cancer prevention and treatment.
© 2013 Japanese Cancer Association.
Figures


References
-
- Simopoulos AP. Genetic variants in the metabolism of omega‐6 and omega‐3 fatty acids: their role in the determination of nutritional requirements and chronic disease risk. Exp Biol Med 2010; 235: 785–95. - PubMed
-
- Innis SM. Dietary (n‐3) fatty acids and brain development. J Nutr 2007; 137: 855–9. - PubMed
-
- Simopoulos AP. Omega‐3 fatty acids in health and disease and in growth and development. Am J Clin Nutr 1991; 54: 438–63. - PubMed
-
- Schaeffer L, Gohlke H, Müller M et al Common genetic variants of the FADS1 FADS2 gene cluster and their reconstructed haplotypes are associated with the fatty acid composition in phospholipids. Hum Mol Genet 2006; 15: 1745–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

