Retention or inversion in stereospecific nickel-catalyzed cross-coupling of benzylic carbamates with arylboronic esters: control of absolute stereochemistry with an achiral catalyst
- PMID: 23414579
- PMCID: PMC3686550
- DOI: 10.1021/ja311783k
Retention or inversion in stereospecific nickel-catalyzed cross-coupling of benzylic carbamates with arylboronic esters: control of absolute stereochemistry with an achiral catalyst
Abstract
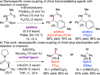
Stereospecific coupling of benzylic carbamates and pivalates with aryl- and heteroarylboronic esters has been developed. The reaction proceeds with selective inversion or retention at the electrophilic carbon, depending on the nature of the ligand. Tricyclohexylphosphine ligand provides the product with retention, while an N-heterocyclic carbene ligand provides the product with inversion.
Figures
References
-
- Lau KSY, Fries RW, Stille JK. J. Am. Chem. Soc. 1974;96:4983.
- Netherton MR, Fu GC. Angew. Chem. Int. Ed. 2002;41:3910. - PubMed
- Legros J-Y, Toffano M, Fiaud J-C. Tetrahedron. 1995;51:3235.
- Rodriquez N, de Arellano CR, Asensio G, Medio-Simon M. Chem. Eur. J. 2007;13:4223. - PubMed
- Lopez-Perez A, Adrio J, Carretero JC. Org. Lett. 2009;11:5514. - PubMed
- He A, Falck JR. J. Am. Chem. Soc. 2010;132:2524. - PMC - PubMed
- Rudolph A, Rackelmann N, Lautens M. Angew. Chem. Int. Ed. 2007;46:1485. - PubMed
-
-
Pd-catalyzed allylic substitutions can occur with inversion or retention, depending on the nucleophile. See: Trost BM, VanVranken DL. Chem. Rev. 1996;96:395.
-
-
- Stille JK, Cowell AB. J. Organomet. Chem. 1977;124:253.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources