Na(+) regulation in the malaria parasite Plasmodium falciparum involves the cation ATPase PfATP4 and is a target of the spiroindolone antimalarials
- PMID: 23414762
- PMCID: PMC3574224
- DOI: 10.1016/j.chom.2012.12.006
Na(+) regulation in the malaria parasite Plasmodium falciparum involves the cation ATPase PfATP4 and is a target of the spiroindolone antimalarials
Abstract
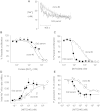
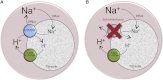
The malaria parasite Plasmodium falciparum establishes in the host erythrocyte plasma membrane new permeability pathways that mediate nutrient uptake into the infected cell. These pathways simultaneously allow Na(+) influx, causing [Na(+)] in the infected erythrocyte cytosol to increase to high levels. The intraerythrocytic parasite itself maintains a low cytosolic [Na(+)] via unknown mechanisms. Here we present evidence that the intraerythrocytic parasite actively extrudes Na(+) against an inward gradient via PfATP4, a parasite plasma membrane protein with sequence similarities to Na(+)-ATPases of lower eukaryotes. Mutations in PfATP4 confer resistance to a potent class of antimalarials, the spiroindolones. Consistent with this, the spiroindolones cause a profound disruption in parasite Na(+) homeostasis, which is attenuated in parasites bearing resistance-conferring mutations in PfATP4. The mutant parasites also show some impairment of Na(+) regulation. Taken together, our results are consistent with PfATP4 being a Na(+) efflux ATPase and a target of the spiroindolones.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures





References
-
- Allen R.J., Kirk K. The membrane potential of the intraerythrocytic malaria parasite Plasmodium falciparum. J. Biol. Chem. 2004;279:11264–11272. - PubMed
-
- Allen R.J., Kirk K. Plasmodium falciparum culture: the benefits of shaking. Mol. Biochem. Parasitol. 2010;169:63–65. - PubMed
-
- Alleva L.M., Kirk K. Calcium regulation in the intraerythrocytic malaria parasite Plasmodium falciparum. Mol. Biochem. Parasitol. 2001;117:121–128. - PubMed
-
- Benito B., Garciadeblás B., Rodríguez-Navarro A. Potassium- or sodium-efflux ATPase, a key enzyme in the evolution of fungi. Microbiology. 2002;148:933–941. - PubMed
-
- Cantley L.C., Jr., Cantley L.G., Josephson L. A characterization of vanadate interactions with the (Na,K)-ATPase. Mechanistic and regulatory implications. J. Biol. Chem. 1978;253:7361–7368. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

