Design of histidine-rich peptides with enhanced bioavailability and inhibitory activity against hepatitis C virus
- PMID: 23415044
- PMCID: PMC7124613
- DOI: 10.1016/j.biomaterials.2013.01.075
Design of histidine-rich peptides with enhanced bioavailability and inhibitory activity against hepatitis C virus
Abstract
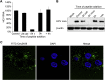
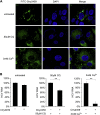
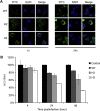
Recently, peptide drugs have evolved into mainstream therapeutics, representing a significant portion of the pharmaceutical market. However, their bioavailability remains to be improved compared with that of chemical drugs. Here, we screened and identified a new peptide, Ctry2459, from a scorpion venom peptide library that was proven to inhibit hepatitis C virus (HCV) infection via inactivating infectious viral particles. However, Ctry2459 cannot suppress established infection of HCV because of the poor cellular uptake and restriction of endosomes. Based on the molecular template of the Ctry2459 peptide, we designed two histidine-rich peptides (Ctry2459-H2 and Ctry2459-H3) with significantly enhanced cellular uptake and improved intracellular distribution. Moreover, the two mutated peptides, as well as the wild-type peptide Ctry2459, exhibited virucidal activities against HCV. In distinct contrast to the Ctry2459 peptide, the mutated peptides significantly suppressed the established HCV infection at the cellular level but demonstrated lower cytotoxic and hemolytic activities. Our work presents an effective design strategy for optimizing natural antiviral peptides and opens a new avenue for enhancing the bioavailability of peptide drugs.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures







References
-
- Katsila T., Siskos A.P., Tamvakopoulos C. Peptide and protein drugs: the study of their metabolism and catabolism by mass spectrometry. Mass Spectrom Rev. 2012;31:110–133. - PubMed
-
- Bidwell G.L. Peptides for cancer therapy: a drug-development opportunity and a drug-delivery challenge. Ther Deliv. 2012;3:609–621. - PubMed
-
- Albericio F., Kruger H.G. Therapeutic peptides. Future Med Chem. 2012;4:1527–1531. - PubMed
-
- Dombu C.Y., Betbeder D. Airway delivery of peptides and proteins using nanoparticles. Biomaterials. 2013;34:516–525. - PubMed
-
- Vlieghe P., Lisowski V., Martinez J., Khrestchatisky M. Synthetic therapeutic peptides: science and market. Drug Discov Today. 2010;15:40–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

