Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS
- PMID: 23415312
- PMCID: PMC3593233
- DOI: 10.1016/j.neuron.2013.02.004
Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS
Abstract
Frontotemporal dementia (FTD) and amyotrophic lateral sclerosis (ALS) are devastating neurodegenerative disorders with clinical, genetic, and neuropathological overlap. Hexanucleotide (GGGGCC) repeat expansions in a noncoding region of C9ORF72 are the major genetic cause of FTD and ALS (c9FTD/ALS). The RNA structure of GGGGCC repeats renders these transcripts susceptible to an unconventional mechanism of translation-repeat-associated non-ATG (RAN) translation. Antibodies generated against putative GGGGCC repeat RAN-translated peptides (anti-C9RANT) detected high molecular weight, insoluble material in brain homogenates, and neuronal inclusions throughout the CNS of c9FTD/ALS cases. C9RANT immunoreactivity was not found in other neurodegenerative diseases, including CAG repeat disorders, or in peripheral tissues of c9FTD/ALS. The specificity of C9RANT for c9FTD/ALS is a potential biomarker for this most common cause of FTD and ALS. These findings have significant implications for treatment strategies directed at RAN-translated peptides and their aggregation and the RNA structures necessary for their production.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

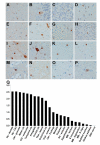
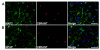

Comment in
-
RANTing about C9orf72.Neuron. 2013 Feb 20;77(4):597-8. doi: 10.1016/j.neuron.2013.02.009. Neuron. 2013. PMID: 23439112
-
Neurodegenerative disease: Researchers identify the protein in c9FTD/ALS inclusions.Nat Rev Neurol. 2013 Apr;9(4):183. doi: 10.1038/nrneurol.2013.39. Epub 2013 Mar 12. Nat Rev Neurol. 2013. PMID: 23478465 No abstract available.
References
-
- Al-Sarraj S, King A, Troakes C, Smith B, Maekawa S, Bodi I, Rogelj B, Al-Chalabi A, Hortobagyi T, Shaw CE. p62 positive, TDP-43 negative, neuronal cytoplasmic and intranuclear inclusions in the cerebellum and hippocampus define the pathology of C9orf72-linked FTLD and MND/ALS. Acta Neuropathol. 2011;122:691–702. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 NS074121-01/NS/NINDS NIH HHS/United States
- P50 NS72187/NS/NINDS NIH HHS/United States
- P50 AG016574/AG/NIA NIH HHS/United States
- R21 NS074121/NS/NINDS NIH HHS/United States
- R01 NS063964/NS/NINDS NIH HHS/United States
- P50 NS072187/NS/NINDS NIH HHS/United States
- R01 ES20395/ES/NIEHS NIH HHS/United States
- R01 ES020395/ES/NIEHS NIH HHS/United States
- P01 AG003949/AG/NIA NIH HHS/United States
- R01 NS080882/NS/NINDS NIH HHS/United States
- R01 AG026251/AG/NIA NIH HHS/United States
- R01 NS077402/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

