11β-Hydroxysteroid dehydrogenase type 1 contributes to the balance between 7-keto- and 7-hydroxy-oxysterols in vivo
- PMID: 23415904
- PMCID: PMC3694296
- DOI: 10.1016/j.bcp.2013.02.002
11β-Hydroxysteroid dehydrogenase type 1 contributes to the balance between 7-keto- and 7-hydroxy-oxysterols in vivo
Abstract
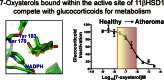
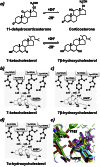
11β-Hydroxysteroid dehydrogenase 1 (11βHSD1; EC 1.1.1.146) generates active glucocorticoids from inert 11-keto metabolites. However, it can also metabolize alternative substrates, including 7β-hydroxy- and 7-keto-cholesterol (7βOHC, 7KC). This has been demonstrated in vitro but its consequences in vivo are uncertain. We used genetically modified mice to investigate the contribution of 11βHSD1 to the balance of circulating levels of 7KC and 7βOHC in vivo, and dissected in vitro the kinetics of the interactions between oxysterols and glucocorticoids for metabolism by the mouse enzyme. Circulating levels of 7KC and 7βOHC in mice were 91.3±22.3 and 22.6±5.7 nM respectively, increasing to 1240±220 and 406±39 nM in ApoE(-/-) mice receiving atherogenic western diet. Disruption of 11βHSD1 in mice increased (p<0.05) the 7KC/7βOHC ratio in plasma (by 20%) and also in isolated microsomes (2 fold). The 7KC/7βOHC ratio was similarly increased when NADPH generation was restricted by disruption of hexose-6-phosphate dehydrogenase. Reduction and oxidation of 7-oxysterols by murine 11βHSD1 proceeded more slowly and substrate affinity was lower than for glucocorticoids. in vitro 7βOHC was a competitive inhibitor of oxidation of corticosterone (Ki=0.9 μM), whereas 7KC only weakly inhibited reduction of 11-dehydrocorticosterone. However, supplementation of 7-oxysterols in cultured cells, secondary to cholesterol loading, preferentially slowed reduction of glucocorticoids, rather than oxidation. Thus, in mouse, 11βHSD1 influenced the abundance and balance of circulating and tissue levels of 7βOHC and 7KC, promoting reduction of 7KC. In health, 7-oxysterols are unlikely to regulate glucocorticoid metabolism. However, in hyperlipidaemia, 7-oxysterols may inhibit glucocorticoid metabolism and modulate signaling through corticosteroid receptors.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

References
-
- Morton N.M., Paterson J.M., Masuzaki H., Holmes M.C., Staels B., Fievet C. Novel adipose tissue-mediated resistance to diet-induced visceral obesity in 11beta-hydroxysteroid dehydrogenase type 1-deficient mice. Diabetes. 2004;53:931–938. - PubMed
-
- Kipari T, Hadoke PWF, Iqbal J, Man TY, Miller E, Coutinho AE. 11β-hydroxysteroid dehydrogenase type 1 deficiency reduces atherosclerosis and plaque inflammation independent of risk factors: key role of the lesional environment. FASEB Journal; http://dx.doi.org/10.1096/fj.12-219105, in press. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

