miR-92b controls glioma proliferation and invasion through regulating Wnt/beta-catenin signaling via Nemo-like kinase
- PMID: 23416699
- PMCID: PMC3635522
- DOI: 10.1093/neuonc/not004
miR-92b controls glioma proliferation and invasion through regulating Wnt/beta-catenin signaling via Nemo-like kinase
Erratum in
- Neuro Oncol. 2013 Jul;15(7):970
Abstract
Background: Nemo-like kinase (NLK) is an evolutionarily conserved protein kinase involved in Wnt/beta-catenin signaling, which has been reported to be associated with gliomagenesis. In the present study, we aimed to identify a concrete mechanism of Wnt/beta-catenin pathway regulation by microRNAs (miRNAs) in glioma.
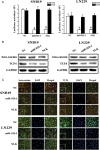
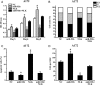
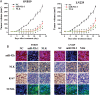
Methods: Quantitative reverse-transcription polymerase chain reaction and in situ hybridization were conducted to detect the expression of miR-92b. The cell proliferation rate and cell cycle kinetics were detected using 3-(4,5)-dimethylthiahiazo (-z-y1)-3,5-di-phenytetrazoliumromide (MTT) assay and flow cytometry, cell invasion and migration were evaluated using Transwell assay and wound healing assay, and cell apoptosis was detected using annexin V staining. Furthermore, the relevant molecules regulating proliferation and invasion were examined using Western blot analysis, immunohistochemistry, and immunofluorescence staining. Luciferase reporter assay was used to identify the direct regulation of NLK by miR-92b and beta-catenin/TCF4 activity.
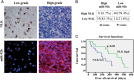
Results: We first showed that the expression of miR-92b was elevated in both glioma samples and glioma cells. Furthermore, down-regulation of miR-92b triggered growth inhibition, induced apoptosis, and suppressed invasion of glioma in vitro and in vivo. Luciferase assay and Western blot analysis revealed that NLK is a direct target of miR-92b. Restoring expression of NLK inhibited glioma proliferation and invasion. Mechanistic investigation revealed that miR-92b deletion suppressed beta-catenin/TCF-4 transcription activity by targeting NLK. Moreover, expression of NLK was inversely correlated with miR-92b in glioma samples and was predictive of patient survival in a retrospective analysis.
Conclusions: Our findings identify a role for miR-92b in glioma proliferation and invasion after activation of Wnt/beta-catenin signaling via NLK.
Figures



References
-
- Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. - PubMed
-
- Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653:1–24. - PubMed
-
- Willert K, Jones KA. Wnt signaling: is the party in the nucleus? Genes Dev. 2006;20:1394–1404. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

