Transcription forms and remodels supercoiling domains unfolding large-scale chromatin structures
- PMID: 23416946
- PMCID: PMC3689368
- DOI: 10.1038/nsmb.2509
Transcription forms and remodels supercoiling domains unfolding large-scale chromatin structures
Abstract
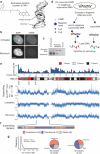
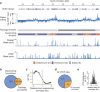
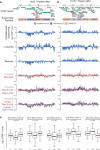
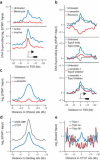
DNA supercoiling is an inherent consequence of twisting DNA and is critical for regulating gene expression and DNA replication. However, DNA supercoiling at a genomic scale in human cells is uncharacterized. To map supercoiling, we used biotinylated trimethylpsoralen as a DNA structure probe to show that the human genome is organized into supercoiling domains. Domains are formed and remodeled by RNA polymerase and topoisomerase activities and are flanked by GC-AT boundaries and CTCF insulator protein-binding sites. Underwound domains are transcriptionally active and enriched in topoisomerase I, 'open' chromatin fibers and DNase I sites, but they are depleted of topoisomerase II. Furthermore, DNA supercoiling affects additional levels of chromatin compaction as underwound domains are cytologically decondensed, topologically constrained and decompacted by transcription of short RNAs. We suggest that supercoiling domains create a topological environment that facilitates gene activation, providing an evolutionary purpose for clustering genes along chromosomes.
Figures



Comment in
-
Genome organization: Supercoiled roles.Nat Rev Genet. 2013 Apr;14(4):238-9. doi: 10.1038/nrg3452. Epub 2013 Mar 5. Nat Rev Genet. 2013. PMID: 23458857 No abstract available.
-
The helix turns at 60: writhing free in chromosomes.Nat Struct Mol Biol. 2013 Mar;20(3):251-3. doi: 10.1038/nsmb.2533. Nat Struct Mol Biol. 2013. PMID: 23463308 No abstract available.
References
-
- Sinden RR, Carlson JO, Pettijohn DE. Torsional tension in the DNA double helix measured with trimethylpsoralen in living E. coli cells: analogous measurements in insect and human cells. Cell. 1980;21:773–783. - PubMed
-
- Villeponteau B, Lundell M, Martinson H. Torsional stress promotes the DNAase I sensitivity of active genes. Cell. 1984;39:469–478. - PubMed
Reference for online methods
-
- Naughton C, et al. Progressive loss of estrogen receptor alpha cofactor recruitment in endocrine resistance. Mol. Endocrinol. 2007;21:2615–2626. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

