Evolution and dynamics of pancreatic cancer progression
- PMID: 23416985
- PMCID: PMC3823715
- DOI: 10.1038/onc.2013.29
Evolution and dynamics of pancreatic cancer progression
Abstract
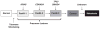
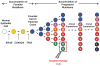
Efficient metastasis is believed as the result of multiple genetic, epigenetic and/or post-translational events in the lifetime of a carcinoma. At the genetic level, these events may be categorized into those that occur during carcinogenesis, and those that occur during subclonal evolution. This review summarizes current knowledge of the genetics of pancreatic cancer from its initiation within a normal cell until the time that is has disseminated to distant organs, many features of which can be extrapolated to other solid tumor types. The implications of these findings to personalize genome analyses of an individual patient's tumor are also discussed.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Nguyen DX, Massagué J. Genetic determinants of cancer metastasis. Nat Rev Genet. 2007;8:341–352. - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. - PubMed
-
- Nguyen DX, Bos PD, Massagué J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. - PubMed
-
- Fidler IJ. The biology of cancer metastasis. Semin Cancer Biol. 2011;21:71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

