Tertiary structure-based analysis of microRNA-target interactions
- PMID: 23417009
- PMCID: PMC3677264
- DOI: 10.1261/rna.035691.112
Tertiary structure-based analysis of microRNA-target interactions
Abstract
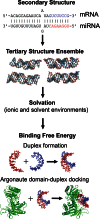
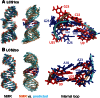
Current computational analysis of microRNA interactions is based largely on primary and secondary structure analysis. Computationally efficient tertiary structure-based methods are needed to enable more realistic modeling of the molecular interactions underlying miRNA-mediated translational repression. We incorporate algorithms for predicting duplex RNA structures, ionic strength effects, duplex entropy and free energy, and docking of duplex-Argonaute protein complexes into a pipeline to model and predict miRNA-target duplex binding energies. To ensure modeling accuracy and computational efficiency, we use an all-atom description of RNA and a continuum description of ionic interactions using the Poisson-Boltzmann equation. Our method predicts the conformations of two constructs of Caenorhabditis elegans let-7 miRNA-target duplexes to an accuracy of ∼3.8 Å root mean square distance of their NMR structures. We also show that the computed duplex formation enthalpies, entropies, and free energies for eight miRNA-target duplexes agree with titration calorimetry data. Analysis of duplex-Argonaute docking shows that structural distortions arising from single-base-pair mismatches in the seed region influence the activity of the complex by destabilizing both duplex hybridization and its association with Argonaute. Collectively, these results demonstrate that tertiary structure-based modeling of miRNA interactions can reveal structural mechanisms not accessible with current secondary structure-based methods.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
