L-type calcium channel targeting and local signalling in cardiac myocytes
- PMID: 23417040
- PMCID: PMC3633156
- DOI: 10.1093/cvr/cvt021
L-type calcium channel targeting and local signalling in cardiac myocytes
Abstract
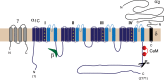
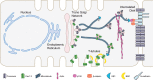
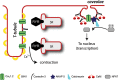
In the heart, Ca(2+) influx via Ca(V)1.2 L-type calcium channels (LTCCs) is a multi-functional signal that triggers muscle contraction, controls action potential duration, and regulates gene expression. The use of LTCC Ca(2+) as a multi-dimensional signalling molecule in the heart is complicated by several aspects of cardiac physiology. Cytosolic Ca(2+) continuously cycles between ~100 nM and ~1 μM with each heartbeat due to Ca(2+) linked signalling from LTCCs to ryanodine receptors. This rapid cycling raises the question as to how cardiac myocytes distinguish the Ca(2+) fluxes originating through L-type channels that are dedicated to contraction from Ca(2+) fluxes originating from other L-type channels that are used for non-contraction-related signalling. In general, disparate Ca(2+) sources in cardiac myocytes such as current through differently localized LTCCs as well as from IP3 receptors can signal selectively to Ca(2+)-dependent effectors in local microdomains that can be impervious to the cytoplasmic Ca(2+) transients that drive contraction. A particular challenge for diversified signalling via cardiac LTCCs is that they are voltage-gated and, therefore, open and presumably flood their microdomains with Ca(2+) with each action potential. Thus spatial localization of Cav1.2 channels to different types of microdomains of the ventricular cardiomyocyte membrane as well as the existence of particular macromolecular complexes in each Cav1.2 microdomain are important to effect different types of Cav1.2 signalling. In this review we examine aspects of Cav1.2 structure, targeting and signalling in two specialized membrane microdomains--transverse tubules and caveolae.
Figures
References
-
- Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi:10.1146/annurev.cellbio.16.1.521. - DOI - PubMed
-
- Tang ZZ, Liang MC, Lu S, Yu D, Yu CY, Yue DT, et al. Transcript scanning reveals novel and extensive splice variations in human l-type voltage-gated calcium channel, Cav1.2 alpha1 subunit. J Biol Chem. 2004;279:44335–44343. doi:10.1074/jbc.M407023200. - DOI - PubMed
-
- Tang ZZ, Hong X, Wang J, Soong TW. Signature combinatorial splicing profiles of rat cardiac- and smooth-muscle Cav1.2 channels with distinct biophysical properties. Cell Calcium. 2007;41:417–428. doi:10.1016/j.ceca.2006.08.002. - DOI - PubMed
-
- De Jongh KS, Murphy BJ, Colvin AA, Hell JW, Takahashi M, Catterall WA. Specific phosphorylation of a site in the full-length form of the alpha 1 subunit of the cardiac L-type calcium channel by adenosine 3′,5′-cyclic monophosphate-dependent protein kinase. Biochemistry. 1996;35:10392–10402. doi:10.1021/bi953023c. - DOI - PubMed
-
- Hulme JT, Yarov-Yarovoy V, Lin TW, Scheuer T, Catterall WA. Autoinhibitory control of the CaV1.2 channel by its proteolytically processed distal C-terminal domain. J Physiol. 2006;576:87–102. doi:10.1113/jphysiol.2006.111799. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

