Structure and interactions of the human programmed cell death 1 receptor
- PMID: 23417675
- PMCID: PMC3636866
- DOI: 10.1074/jbc.M112.448126
Structure and interactions of the human programmed cell death 1 receptor
Abstract
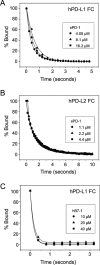
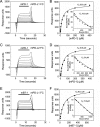
PD-1, a receptor expressed by T cells, B cells, and monocytes, is a potent regulator of immune responses and a promising therapeutic target. The structure and interactions of human PD-1 are, however, incompletely characterized. We present the solution nuclear magnetic resonance (NMR)-based structure of the human PD-1 extracellular region and detailed analyses of its interactions with its ligands, PD-L1 and PD-L2. PD-1 has typical immunoglobulin superfamily topology but differs at the edge of the GFCC' sheet, which is flexible and completely lacks a C" strand. Changes in PD-1 backbone NMR signals induced by ligand binding suggest that, whereas binding is centered on the GFCC' sheet, PD-1 is engaged by its two ligands differently and in ways incompletely explained by crystal structures of mouse PD-1 · ligand complexes. The affinities of these interactions and that of PD-L1 with the costimulatory protein B7-1, measured using surface plasmon resonance, are significantly weaker than expected. The 3-4-fold greater affinity of PD-L2 versus PD-L1 for human PD-1 is principally due to the 3-fold smaller dissociation rate for PD-L2 binding. Isothermal titration calorimetry revealed that the PD-1/PD-L1 interaction is entropically driven, whereas PD-1/PD-L2 binding has a large enthalpic component. Mathematical simulations based on the biophysical data and quantitative expression data suggest an unexpectedly limited contribution of PD-L2 to PD-1 ligation during interactions of activated T cells with antigen-presenting cells. These findings provide a rigorous structural and biophysical framework for interpreting the important functions of PD-1 and reveal that potent inhibitory signaling can be initiated by weakly interacting receptors.
Figures




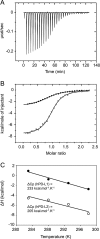
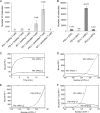
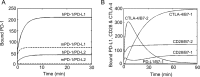
References
-
- Nishimura H., Nose M., Hiai H., Minato N., Honjo T. (1999) Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 11, 141–151 - PubMed
-
- Nishimura H., Okazaki T., Tanaka Y., Nakatani K., Hara M., Matsumori A., Sasayama S., Mizoguchi A., Hiai H., Minato N., Honjo T. (2001) Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 291, 319–322 - PubMed
-
- Ferreiros-Vidal I., Gomez-Reino J. J., Barros F., Carracedo A., Carreira P., Gonzalez-Escribano F., Liz M., Martin J., Ordi J., Vicario J. L., Gonzalez A. (2004) Association of PDCD1 with susceptibility to systemic lupus erythematosus. Evidence of population-specific effects. Arthritis Rheum. 50, 2590–2597 - PubMed
-
- James E. S., Harney S., Wordsworth B. P., Cookson W. O., Davis S. J., Moffatt M. F. (2005) PDCD1. A tissue-specific susceptibility locus for inherited inflammatory disorders. Genes Immun. 6, 430–437 - PubMed
-
- Kong E. K., Prokunina-Olsson L., Wong W. H., Lau C. S., Chan T. M., Alarcón-Riquelme M., Lau Y. L. (2005) A new haplotype of PDCD1 is associated with rheumatoid arthritis in Hong Kong Chinese. Arthritis Rheum. 52, 1058–1062 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

