Hydroxyethyl starch 130/0.38-0.45 versus crystalloid or albumin in patients with sepsis: systematic review with meta-analysis and trial sequential analysis
- PMID: 23418281
- PMCID: PMC3573769
- DOI: 10.1136/bmj.f839
Hydroxyethyl starch 130/0.38-0.45 versus crystalloid or albumin in patients with sepsis: systematic review with meta-analysis and trial sequential analysis
Abstract
Objective: To assess the effects of fluid therapy with hydroxyethyl starch 130/0.38-0.45 versus crystalloid or albumin on mortality, kidney injury, bleeding, and serious adverse events in patients with sepsis.
Design: Systematic review with meta-analyses and trial sequential analyses of randomised clinical trials.
Data sources: Cochrane Library, Medline, Embase, Biosis Previews, Science Citation Index Expanded, CINAHL, Current Controlled Trials, Clinicaltrials.gov, and Centerwatch to September 2012; hand search of reference lists and other systematic reviews; contact with authors and relevant pharmaceutical companies.
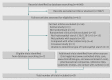
Study selection: Eligible trials were randomised clinical trials comparing hydroxyethyl starch 130/0.38-0.45 with either crystalloid or human albumin in patients with sepsis. Published and unpublished trials were included irrespective of language and predefined outcomes.
Data extraction: Two reviewers independently assessed studies for inclusion and extracted data on methods, interventions, outcomes, and risk of bias. Risk ratios and mean differences with 95% confidence intervals were estimated with fixed and random effects models.
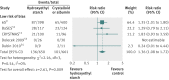
Results: Nine trials that randomised 3456 patients with sepsis were included. Overall, hydroxyethyl starch 130/0.38-0.45 versus crystalloid or albumin did not affect the relative risk of death (1.04, 95% confidence interval 0.89 to 1.22, 3414 patients, eight trials), but in the predefined analysis of trials with low risk of bias the relative risk of death was 1.11 (1.00 to 1.23, trial sequential analysis (TSA) adjusted 95% confidence interval 0.95 to 1.29, 3016 patients, four trials). In the hydroxyethyl starch group, renal replacement therapy was used more (1.36, 1.08 to 1.72, TSA adjusted 1.03 to 1.80, 1311 patients, five trials), and the relative risk of acute kidney injury was 1.18 (0.99 to 1.40, TSA adjusted 0.90 to 1.54, 994 patients, four trials). More patients in the hydroxyethyl starch group were transfused with red blood cells (1.29, 1.13 to 1.48, TSA adjusted 1.10 to 1.51, 973 patients, three trials), and more patients had serious adverse events (1.30, 1.02 to 1.67, TSA adjusted 0.93 to 1.83, 1069 patients, four trials). The transfused volume of red blood cells did not differ between the groups (mean difference 65 mL, 95% confidence interval -20 to 149 mL, three trials).
Conclusion: In conventional meta-analyses including recent trial data, hydroxyethyl starch 130/0.38-0.45 versus crystalloid or albumin increased the use of renal replacement therapy and transfusion with red blood cells, and resulted in more serious adverse events in patients with sepsis. It seems unlikely that hydroxyethyl starch 130/0.38-0.45 provides overall clinical benefit for patients with sepsis.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures



Comment in
-
Is it the end of the road for synthetic starches in critical illness? No place for hydroxyethyl starch solutions in treatment of patients with sepsis.BMJ. 2013 Mar 20;346:f1805. doi: 10.1136/bmj.f1805. BMJ. 2013. PMID: 23516156 No abstract available.
References
-
- Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008;358:125-39. - PubMed
-
- Schortgen F, Lacherade JC, Bruneel F, Cattaneo I, Hemery F, Lemaire F, et al. Effects of hydroxyethylstarch and gelatin on renal function in severe sepsis: a multicentre randomised study. Lancet 2001;357:911-6. - PubMed
-
- Hartog CS, Kohl M, Reinhart K. A systematic review of third-generation hydroxyethyl starch (HES 130/0.4) in resuscitation: safety not adequately addressed. Anesth Analg 2011;112:635-45. - PubMed
-
- Dart AB, Mutter TC, Ruth CA, Taback SP. Hydroxyethyl starch (HES) versus other fluid therapies: effects on kidney function. Cochrane Database Syst Rev 2010;(1):CD007594. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
