Cells derived from the coelomic epithelium contribute to multiple gastrointestinal tissues in mouse embryos
- PMID: 23418471
- PMCID: PMC3572158
- DOI: 10.1371/journal.pone.0055890
Cells derived from the coelomic epithelium contribute to multiple gastrointestinal tissues in mouse embryos
Abstract
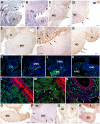
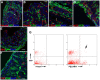
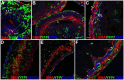
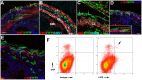
Gut mesodermal tissues originate from the splanchnopleural mesenchyme. However, the embryonic gastrointestinal coelomic epithelium gives rise to mesenchymal cells, whose significance and fate are little known. Our aim was to investigate the contribution of coelomic epithelium-derived cells to the intestinal development. We have used the transgenic mouse model mWt1/IRES/GFP-Cre (Wt1(cre)) crossed with the Rosa26R-EYFP reporter mouse. In the gastrointestinal duct Wt1, the Wilms' tumor suppressor gene, is specific and dynamically expressed in the coelomic epithelium. In the embryos obtained from the crossbreeding, the Wt1-expressing cell lineage produces the yellow fluorescent protein (YFP) allowing for colocalization with differentiation markers through confocal microscopy and flow cytometry. Wt1(cre-YFP) cells were very abundant throughout the intestine during midgestation, declining in neonates. Wt1(cre-YFP) cells were also transiently observed within the mucosa, being apparently released into the intestinal lumen. YFP was detected in cells contributing to intestinal vascularization (endothelium, pericytes and smooth muscle), visceral musculature (circular, longitudinal and submucosal) as well as in Cajal and Cajal-like interstitial cells. Wt1(cre-YFP) mesenchymal cells expressed FGF9, a critical growth factor for intestinal development, as well as PDGFRα, mainly within developing villi. Thus, a cell population derived from the coelomic epithelium incorporates to the gut mesenchyme and contribute to a variety of intestinal tissues, probably playing also a signaling role. Our results support the origin of interstitial cells of Cajal and visceral circular muscle from a common progenitor expressing anoctamin-1 and SMCα-actin. Coelomic-derived cells contribute to the differentiation of at least a part of the interstitial cells of Cajal.
Conflict of interest statement
Figures

References
-
- Rolle U, Piaseczna-Piotrowska A, Puri P (2007) Interstitial cells of Cajal in the normal gut and in intestinal motility disorders of childhood. Pediatr Surg Int 23: 1139–1152. - PubMed
-
- Lavine KJ, Yu K, White AC, Zhang X, Smith C, et al. (2005) Endocardial and epicardial derived FGF signals regulate myocardial proliferation and differentiation in vivo. Dev Cell 8: 85–95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

