Changes in the Secretory Profile of NSCLC-Associated Fibroblasts after Ablative Radiotherapy: Potential Impact on Angiogenesis and Tumor Growth
- PMID: 23418618
- PMCID: PMC3573655
- DOI: 10.1593/tlo.12349
Changes in the Secretory Profile of NSCLC-Associated Fibroblasts after Ablative Radiotherapy: Potential Impact on Angiogenesis and Tumor Growth
Abstract
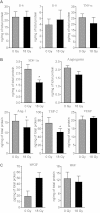
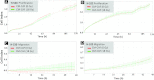
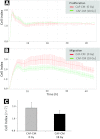
In the context of radiotherapy, collateral effects of ablative doses of ionizing radiation (AIR) on stromal components of tumors remains understudied. In this work, cancer-associated fibroblasts (CAFs) isolated from freshly resected human lung tumors were exposed to AIR (1x 18 Gy) and analyzed for their release of paracrine factors. Inflammatory mediators and regulators of angiogenesis and tumor growth were analyzed by multiplex protein assays in conditioned medium (CM) from irradiated and non-irradiated CAFs. Additionally, the profile of secreted proteins was examined by proteomics. In functional assays, effects of CAF-CM on proliferative and migratory capacity of lung tumor cells (H-520/H-522) and human umbilical vein endothelial cells (HUVECs) and their tube-forming capacity were assessed. Our data show that exposure of CAFs to AIR results in 1) downregulated release of angiogenic molecules such as stromal cell-derived factor-1, angiopoietin, and thrombospondin-2 (TSP-2); 2) upregulated release of basic fibroblast growth factor from most donors; and 3) unaffected expression levels of hepatocyte growth factor, interleukin-6 (IL-6), IL-8, IL-1β, and tumor necrosis factor-α. CM from irradiated and control CAFs did not affect differently the proliferative or migratory capacity of tumor cells (H-520/H-522), whereas migratory capacity of HUVECs was partially reduced in the presence of irradiated CAF-CM. Overall, we conclude that AIR mediates a transformation on the secretory profile of CAFs that could influence the behavior of other cells in the tumor tissue and hence guide therapeutic outcomes. Downstream consequences of the changes observed in this study merits further investigations.
Figures

References
-
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. - PubMed
-
- Powell JW, Dexter E, Scalzetti EM, Bogart JA. Treatment advances for medically inoperable non-small-cell lung cancer: emphasis on prospective trials. Lancet Oncol. 2009;10:885–894. - PubMed
-
- Kavanagh BD, Miften M, Rabinovitch RA. Advances in treatment techniques: stereotactic body radiation therapy and the spread of hypofractionation. Cancer J. 2011;17:177–181. - PubMed
-
- Heinzerling JH, Kavanagh B, Timmerman RD. Stereotactic ablative radiation therapy for primary lung tumors. Cancer J. 2011;17:28–32. - PubMed
-
- Lanni TB, Jr, Grills IS, Kestin LL, Robertson JM. Stereotactic radiotherapy reduces treatment cost while improving overall survival and local control over standard fractionated radiation therapy for medically inoperable non-small-cell lung cancer. Am J Clin Oncol. 2011;34:494–498. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
