Opioid peptidomimetics: leads for the design of bioavailable mixed efficacy μ opioid receptor (MOR) agonist/δ opioid receptor (DOR) antagonist ligands
- PMID: 23419026
- PMCID: PMC3618660
- DOI: 10.1021/jm400050y
Opioid peptidomimetics: leads for the design of bioavailable mixed efficacy μ opioid receptor (MOR) agonist/δ opioid receptor (DOR) antagonist ligands
Abstract
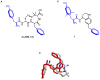
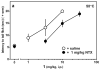
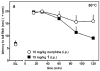
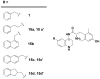
We have previously described opioid peptidomimetic, 1, employing a tetrahydroquinoline scaffold and modeled on a series of cyclic tetrapeptide opioid agonists. We have recently described modifications to these peptides that confer a μ opioid receptor (MOR) agonist, δ opioid receptor (DOR) antagonist profile, which has been shown to reduce the development of tolerance to the analgesic actions of MOR agonists. Several such bifunctional ligands have been reported, but none has been demonstrated to cross the blood-brain barrier. Here we describe the transfer of structural features that evoked MOR agonist/DOR antagonist behavior in the cyclic peptides to the tetrahydroquinoline scaffold and show that the resulting peptidomimetics maintain the desired pharmacological profile. Further, the 4R diastereomer of 1 was fully efficacious and approximately equipotent to morphine in the mouse warm water tail withdrawal assay following intraperitoneal administration and thus a promising lead for the development of opioid analgesics with reduced tolerance.
Figures
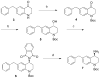

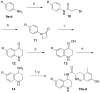
References
-
- Morphy R, Kay C, Rankovic Z. From Magic Bullets to Designed Multiple Ligands. Res Focus Rev. 2004;9:641–652. - PubMed
-
- Morphy R, Rankovic Z. Designing Multiple Ligands - Medicinal Chemistry Strategies and Challenges. Curr Pharmaceut Des. 2009;15:587–600. - PubMed
-
- Morphy R, Rankovic Z. Designed multiple ligands. An emerging drug discovery paradigm. J Med Chem. 2005;48:6523–6543. - PubMed
-
- Abdelhamid EE, Sultana M, Portoghese PS, Takemori AE. Selective Blockage of the Delta Opioid Receptors Prevents the Development of Morphine Tolerance and Dependence in Mice. J Pharmacol Exp Ther. 1991;258:299–303. - PubMed
-
- Fundytus ME, Schiller PW, Shapiro M, Weltrowska G, Coderre TJ. Attenuation of Morphine Tolerance and Dependence with the Highly Selective Delta Opioid Receptor Antagonist TIPP(psi) Eur J Pharmacol. 1995;286:105–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Research Materials

