In vitro engineering of vascularized tissue surrogates
- PMID: 23419835
- PMCID: PMC3575583
- DOI: 10.1038/srep01316
In vitro engineering of vascularized tissue surrogates
Abstract
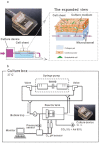
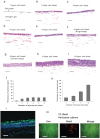
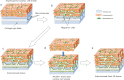
In vitro scaling up of bioengineered tissues is known to be limited by diffusion issues, specifically a lack of vasculature. Here, we report a new strategy for preserving cell viability in three-dimensional tissues using cell sheet technology and a perfusion bioreactor having collagen-based microchannels. When triple-layer cardiac cell sheets are incubated within this bioreactor, endothelial cells in the cell sheets migrate to vascularize in the collagen gel, and finally connect with the microchannels. Medium readily flows into the cell sheets through the microchannels and the newly developed capillaries, while the cardiac construct shows simultaneous beating. When additional triple-layer cell sheets are repeatedly layered, new multi-layer construct spontaneously integrates and the resulting construct becomes a vascularized thick tissue. These results confirmed our method to fabricate in vitro vascularized tissue surrogates that overcomes engineered-tissue thickness limitations. The surrogates promise new therapies for damaged organs as well as new in vitro tissue models.
Conflict of interest statement
T. Shimizu and M. Yamato are consultants for CellSeed, Inc. T. Okano is an investor in CellSeed, Inc., and is an inventor/developer designated on the patent for temperature-responsive culture surfaces.
Figures



References
-
- Levenberg S. et al. Engineering vascularized skeletal muscle tissue. Nature Biotechnology 23, 879–884 (2005). - PubMed
-
- Morritt A. N. et al. Cardiac tissue engineering in an in vivo vascularized chamber. Circulation 115, 353–360 (2007). - PubMed
-
- Shimizu T. et al. Fabrication of pulsatile cardiac tissue grafts using a novel 3-dimensional cell sheet manipulation technique and temperature-responsive cell culture surfaces. Circulation Research 90, e40–e48 (2002). - PubMed
-
- Shimizu T. et al. Polysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissues. The FASEB Journal 20, 708 (2006). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

