Diacylglycerol acyltransferase-1 localizes hepatitis C virus NS5A protein to lipid droplets and enhances NS5A interaction with the viral capsid core
- PMID: 23420847
- PMCID: PMC3617291
- DOI: 10.1074/jbc.M112.434910
Diacylglycerol acyltransferase-1 localizes hepatitis C virus NS5A protein to lipid droplets and enhances NS5A interaction with the viral capsid core
Erratum in
-
Correction: Diacylglycerol acyltransferase-1 localizes hepatitis C virus NS5A protein to lipid droplets and enhances NS5A interaction with the viral capsid core.J Biol Chem. 2024 Dec;300(12):107975. doi: 10.1016/j.jbc.2024.107975. Epub 2024 Nov 30. J Biol Chem. 2024. PMID: 39616949 Free PMC article. No abstract available.
Abstract
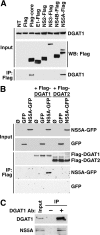
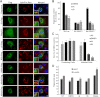
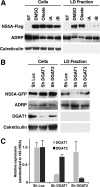
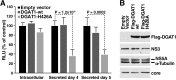
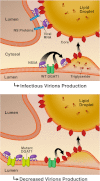
The triglyceride-synthesizing enzyme acyl CoA:diacylglycerol acyltransferase 1 (DGAT1) plays a critical role in hepatitis C virus (HCV) infection by recruiting the HCV capsid protein core onto the surface of cellular lipid droplets (LDs). Here we find a new interaction between the non-structural protein NS5A and DGAT1 and show that the trafficking of NS5A to LDs depends on DGAT1 activity. DGAT1 forms a complex with NS5A and core and facilitates the interaction between both viral proteins. A catalytically inactive mutant of DGAT1 (H426A) blocks the localization of NS5A, but not core, to LDs in a dominant-negative manner and impairs the release of infectious viral particles, underscoring the importance of DGAT1-mediated translocation of NS5A to LDs in viral particle production. We propose a model whereby DGAT1 serves as a cellular hub for HCV core and NS5A proteins, guiding both onto the surface of the same subset of LDs, those generated by DGAT1. These results highlight the critical role of DGAT1 as a host factor for HCV infection and as a potential drug target for antiviral therapy.
Figures



References
-
- Wasley A., Alter M. J. (2000) Epidemiology of hepatitis C. Geographic differences and temporal trends. Semin. Liver Dis. 20, 1–16 - PubMed
-
- Feld J. J., Hoofnagle J. H. (2005) Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature 436, 967–972 - PubMed
-
- Masaki T., Suzuki R., Murakami K., Aizaki H., Ishii K., Murayama A., Date T., Matsuura Y., Miyamura T., Wakita T., Suzuki T. (2008) Interaction of hepatitis C virus nonstructural protein 5A with core protein is critical for the production of infectious virus particles. J. Virol. 82, 7964–7976 - PMC - PubMed
-
- Blight K. J., Kolykhalov A. A., Rice C. M. (2000) Efficient initiation of HCV RNA replication in cell culture. Science 290, 1972–1974 - PubMed
-
- Miyanari Y., Atsuzawa K., Usuda N., Watashi K., Hishiki T., Zayas M., Bartenschlager R., Wakita T., Hijikata M., Shimotohno K. (2007) The lipid droplet is an important organelle for hepatitis C virus production. Nat. Cell Biol. 9, 1089–1097 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

