Biochemical properties of a novel thermostable and highly xylose-tolerant β-xylosidase/α-arabinosidase from Thermotoga thermarum
- PMID: 23422003
- PMCID: PMC3621209
- DOI: 10.1186/1754-6834-6-27
Biochemical properties of a novel thermostable and highly xylose-tolerant β-xylosidase/α-arabinosidase from Thermotoga thermarum
Abstract
Background: β-Xylosidase is an important constituent of the hemicellulase system and it plays an important role in hydrolyzing xylooligosaccharides to xylose. Xylose, a useful monose, has been utilized in a wide range of applications such as food, light, chemical as well as energy industry. Therefore, the xylose-tolerant β-xylosidase with high specific activity for bioconversion of xylooligosaccharides has a great potential in the fields as above.
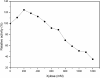
Results: A β-xylosidase gene (Tth xynB3) of 2,322 bp was cloned from the extremely thermophilic bacterium Thermotoga thermarum DSM 5069 that encodes a protein containing 774 amino acid residues, and was expressed in Escherichia coli BL21 (DE3). The phylogenetic trees of β-xylosidases were constructed using Neighbor-Joining (NJ) and Maximum-Parsimony (MP) methods. The phylogeny and amino acid analysis indicated that the Tth xynB3 β-xylosidase was a novel β-xylosidase of GH3. The optimal activity of the Tth xynB3 β-xylosidase was obtained at pH 6.0 and 95°C and was stable over a pH range of 5.0-7.5 and exhibited 2 h half-life at 85°C. The kinetic parameters Km and Vmax values for p-nitrophenyl-β-D-xylopyranoside and p-nitrophenyl-α-L-arabinofuranoside were 0.27 mM and 223.3 U/mg, 0.21 mM and 75 U/mg, respectively. The kcat/Km values for p-nitrophenyl-β-D-xylopyranoside and p-nitrophenyl-α-L-arabinofuranoside were 1,173.4 mM-1 s-1 and 505.9 mM-1 s-1, respectively. It displayed high tolerance to xylose, with Ki value approximately 1000 mM. It was stimulated by xylose at higher concentration up to 500 mM, above which the enzyme activity of Tth xynB3 β-xylosidase was gradually decreased. However, it still remained approximately 50% of its original activity even if the concentration of xylose was as high as 1000 mM. It was also discovered that the Tth xynB3 β-xylosidase exhibited a high hydrolytic activity on xylooligosaccharides. When 5% substrate was incubated with 0.3 U Tth xynB3 β-xylosidase in 200 μL reaction system for 3 h, almost all the substrate was biodegraded into xylose.
Conclusions: The article provides a useful and novel β-xylosidase displaying extraordinary and desirable properties: high xylose tolerance and catalytic activity at temperatures above 75°C, thermally stable and excellent hydrolytic activity on xylooligosaccharides.
Figures

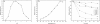


References
-
- Quintero D, Velasco Z, Hurtado-Gómez E, Neira JL, Contreras LM. Isolation and characterization of a thermostable β-xylosidase in the thermophilic bacterium Geobacillus pallidus. Biochimica et Biophysica Acta (BBA) - Proteins & Proteomics. 2007;1774(4):510–518. doi: 10.1016/j.bbapap.2007.02.002. - DOI - PubMed
-
- Kambourova M, Mandeva R, Fiume I, Maurelli L, Rossi M, Morana A. Hydrolysis of xylan at high temperature by co-action of the xylanase from Anoxybacillus flavithermus BC and the β-xylosidase/α-arabinosidase from Sulfolobus solfataricus Oα. J Appl Microbiol. 2007;102(6):1586–1593. doi: 10.1111/j.1365-2672.2006.03197.x. - DOI - PubMed
-
- Morana A, Paris O, Maurelli L, Rossi M, Cannio R. Gene cloning and expression in Escherichia coli of a bi-functional β-D-xylosidase/α-L-arabinosidase from Sulfolobus solfataricus involved in xylan degradation. Extremophiles. 2006;11(1):123–132. - PubMed
-
- Bokhari SAI, Latif F, Akhtar MW, Rajoka MI. Characterization of a β-xylosidase produced by a mutant derivative of Humicola lanuginosa in solid state fermentation. Annals of Microbiology. 2010;60(1):21–29. doi: 10.1007/s13213-010-0026-3. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

