A quorum-sensing-induced bacteriophage defense mechanism
- PMID: 23422409
- PMCID: PMC3624510
- DOI: 10.1128/mBio.00362-12
A quorum-sensing-induced bacteriophage defense mechanism
Abstract
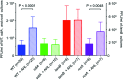
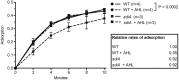
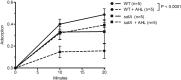
One of the key determinants of the size, composition, structure, and development of a microbial community is the predation pressure by bacteriophages. Accordingly, bacteria have evolved a battery of antiphage defense strategies. Since maintaining constantly elevated defenses is costly, we hypothesize that some bacteria have additionally evolved the abilities to estimate the risk of phage infection and to adjust their strategies accordingly. One risk parameter is the density of the bacterial population. Hence, quorum sensing, i.e., the ability to regulate gene expression according to population density, may be an important determinant of phage-host interactions. This hypothesis was investigated in the model system of Escherichia coli and phage λ. We found that, indeed, quorum sensing constitutes a significant, but so far overlooked, determinant of host susceptibility to phage attack. Specifically, E. coli reduces the numbers of λ receptors on the cell surface in response to N-acyl-l-homoserine lactone (AHL) quorum-sensing signals, causing a 2-fold reduction in the phage adsorption rate. The modest reduction in phage adsorption rate leads to a dramatic increase in the frequency of uninfected survivor cells after a potent attack by virulent phages. Notably, this mechanism may apply to a broader range of phages, as AHLs also reduce the risk of χ phage infection through a different receptor. IMPORTANCE To enable the successful manipulation of bacterial populations, a comprehensive understanding of the factors that naturally shape microbial communities is required. One of the key factors in this context is the interactions between bacteria and the most abundant biological entities on Earth, namely, the bacteriophages that prey on bacteria. This proof-of-principle study shows that quorum sensing plays an important role in determining the susceptibility of E. coli to infection by bacteriophages λ and χ. On the basis of our findings in the classical Escherichia coli-λ model system, we suggest that quorum sensing may serve as a general strategy to protect bacteria specifically under conditions of high risk of infection.
Figures


References
-
- Brüssow H, Hendrix RW. 2002. Phage genomics: small is beautiful. Cell 108:13–16 - PubMed
-
- Labrie SJ, Samson JE, Moineau S. 2010. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 8:317–327 - PubMed
-
- Gómez P, Buckling A. 2011. Bacteria-phage antagonistic coevolution in soil. Science 332:106–109 - PubMed
-
- Hall AR, Scanlan PD, Morgan AD, Buckling A. 2011. Host-parasite coevolutionary arms races give way to fluctuating selection. Ecol. Lett. 14:635–642 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
