A ring-distortion strategy to construct stereochemically complex and structurally diverse compounds from natural products
- PMID: 23422561
- PMCID: PMC3965367
- DOI: 10.1038/nchem.1549
A ring-distortion strategy to construct stereochemically complex and structurally diverse compounds from natural products
Abstract
High-throughput screening is the dominant method used to identify lead compounds in drug discovery. As such, the makeup of screening libraries largely dictates the biological targets that can be modulated and the therapeutics that can be developed. Unfortunately, most compound-screening collections consist principally of planar molecules with little structural or stereochemical complexity, compounds that do not offer the arrangement of chemical functionality necessary for the modulation of many drug targets. Here we describe a novel, general and facile strategy for the creation of diverse compounds with high structural and stereochemical complexity using readily available natural products as synthetic starting points. We show through the evaluation of chemical properties (which include fraction of sp(3) carbons, ClogP and the number of stereogenic centres) that these compounds are significantly more complex and diverse than those in standard screening collections, and we give guidelines for the application of this strategy to any suitable natural product.
Conflict of interest statement
Figures

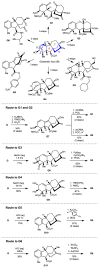
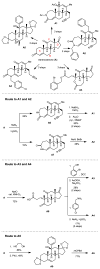
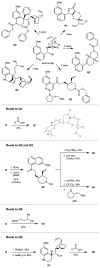

Comment in
-
Drug discovery: Diversifying complexity.Nat Chem. 2013 Mar;5(3):157-8. doi: 10.1038/nchem.1581. Nat Chem. 2013. PMID: 23422555 No abstract available.
References
-
- Swinney DC, Anthony J. How were new medicines discovered? Nat Rev Drug Discov. 2011;10:507–519. - PubMed
-
- Flaherty KT, Yasothan U, Kirkpatrick P. Vemurafenib. Nat Rev Drug Discov. 2011;10:811–812. - PubMed
-
- Domling A. Small molecular weight protein-protein interaction antagonists: an insurmountable challenge? Curr Opin Chem Biol. 2008;12:281–291. - PubMed
-
- Grivas PD, Kiaris H, Papavassiliou AG. Tackling transcription factors: challenges in antitumor therapy. Trends Mol Med. 2011;17:537–538. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

